




















中国农业科技导报 ›› 2025, Vol. 27 ›› Issue (10): 10-23.DOI: 10.13304/j.nykjdb.2024.0290
• 农业创新论坛 • 上一篇
赵雄( ), 马关柳, 郭通琴, 何国菊, 赵许朋, 刘声传(
), 马关柳, 郭通琴, 何国菊, 赵许朋, 刘声传( )
)
收稿日期:2024-04-12
接受日期:2024-09-18
出版日期:2025-10-15
发布日期:2025-10-15
通讯作者:
刘声传
作者简介:赵雄E-mail: zhaox@gyu.cn;
基金资助:
Xiong ZHAO( ), Guanliu MA, Tongqin GUO, Guoju HE, Xupeng ZHAO, Shengchuan LIU(
), Guanliu MA, Tongqin GUO, Guoju HE, Xupeng ZHAO, Shengchuan LIU( )
)
Received:2024-04-12
Accepted:2024-09-18
Online:2025-10-15
Published:2025-10-15
Contact:
Shengchuan LIU
摘要:
表皮蜡质是覆盖在陆地植物地上部分最外层的一层疏水性保护屏障,在植物抵御生物和非生物胁迫等方面起重要作用。模式植物表皮蜡质生物合成转运途径已被逐渐揭示,一些结构基因和调控基因相继被发掘和鉴定,但其合成转运的分子调控机制仍不很清楚,且在非模式植物中的研究较少。总结了植物表皮蜡质结构、组分和功能,并阐述了植物表皮蜡质的生物合成和转运机制、相关结构基因的功能,重点梳理了植物表皮蜡质生物合成的转录、转录后、翻译后和表观遗传调控机制,并对今后研究重点进行展望,以期为植物表皮蜡质的后续研究与农业生产实践提供启示。
中图分类号:
赵雄, 马关柳, 郭通琴, 何国菊, 赵许朋, 刘声传. 植物表皮蜡质生物合成及其分子调控研究进展[J]. 中国农业科技导报, 2025, 27(10): 10-23.
Xiong ZHAO, Guanliu MA, Tongqin GUO, Guoju HE, Xupeng ZHAO, Shengchuan LIU. Recent Advances on Cuticular Wax Biosynthesis and Its Molecular Regulation in Plants[J]. Journal of Agricultural Science and Technology, 2025, 27(10): 10-23.
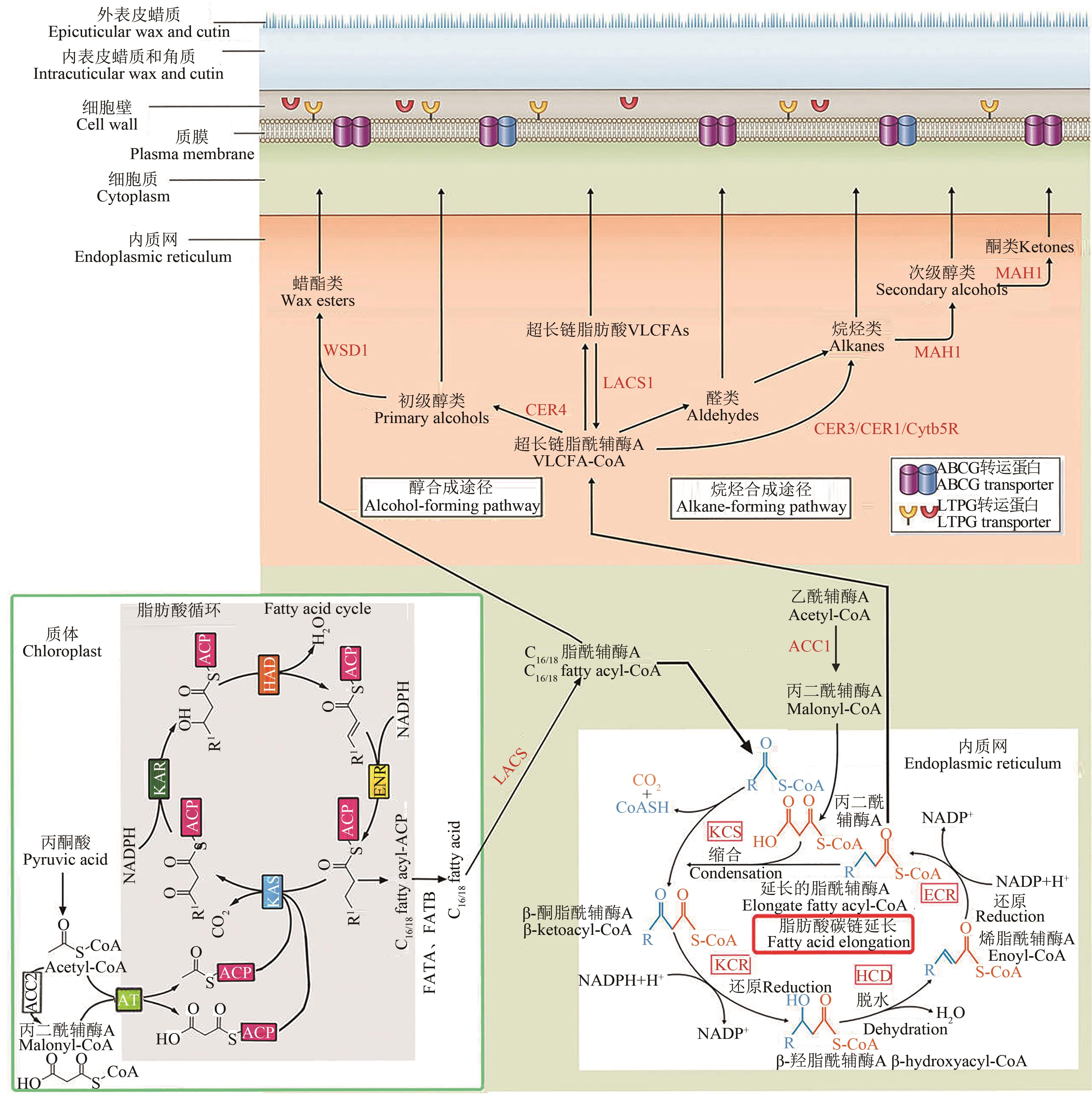
图2 植物表皮蜡质生物合成与转运途径[2,17]注:ABCG—ATP结合盒G转运蛋白;ACC 1/2—乙酰辅酶A羧化酶1/2;ACP—酰基载体蛋白;AT—脂酰基转移酶;CER1/3/4—脂酰辅酶A还原酶1/2/3;Cytb5R—细胞色素b5还原酶;ECR—烯脂酰-辅酶A还原酶;ENR—烯脂酰-ACP还原酶;FATA/FATB—脂酰-ACP硫酯酶A/B;HAD—β-羟脂酰-ACP脱水酶;HCD—β-羟脂酰-辅酶A脱水酶;KAR—β-酮脂酰-ACP还原酶;KAS—β-酮脂酰-ACP合成酶;KCR—β-酮脂酰-辅酶A还原酶;KCS—β-酮脂酰-辅酶A合成酶;LACS—长链酰基辅酶A合成酶;LTPG—糖基磷脂酰肌醇锚定脂质转运蛋白;MAH1—中链烷烃羟化酶1;WSD1—蜡酯合成酶1。R1或R表示增长的烷基链。
Fig. 2 Plant cuticular wax biosynthesis and transport pathway[2,17]Note:ABCG—ATP-binding cassette subfamily G; ACC 1/2—Acetyl-coenzyme A (CoA) carboxylase 1/2; ACP—Acyl carrier protein;AT— Acyltransferase; CER1/3/4—ECERIFERUM1/3/4; Cytb5R—Cytochrome b5 reductase;ECR—Enoyl-CoA reductase;ENR—Enoyl-ACP reductase; FATA/FATB—Fatty acyl-ACP thioesterase A/B;HAD—β-hydroxyacyl-ACP dehydratase;HCD—β-hydroxyacyl-CoA dehydratase; KAR—β-ketoacyl-ACP reductase; KAS—β-ketoacyl-ACP synthase; KCR—β-ketoacyl-CoA reductase; KCS—β-ketoacyl-CoA synthase; LACS—Long-chain-acyl-CoA synthetase; LTPG—Glycosylphosphatidylinositol-anchored lipid transfer protein; MAH1—Midchain alkane hydroxylase 1; WSD1—Wax ester synthase 1. R1 or R refers to the growing alkyl chain wax ester synthase/diacylglycerol acyltransferase 1.
物种 Species | 基因名 Gene name | 功能 Function | 参考文献 Reference |
|---|---|---|---|
| 拟南芥Arabidopsis thaliana | CER17 | 超长链酰基辅酶A的n-6去饱和 n-6 desaturation of very long chain acyl-CoAs | [ |
| 拟南芥Arabidopsis thaliana | LACS1, LACS2, LACS4 | 蜡质合成过程中重叠 Overlap in wax synthesis | [ |
| 拟南芥Arabidopsis thaliana | Cytb5R | CER1的辅助因子Cofactor of CER1 | [ |
| 拟南芥Arabidopsis thaliana | WAX2/CER3 | 醛类合成Synthesis of aldehydes | [ |
| 拟南芥Arabidopsis thaliana | ABCG11/WBC11,ABCG12/CER5 | 蜡质转运Cuticular wax transport | [ |
| 拟南芥Arabidopsis thaliana | ACC1 | 丙二酰辅酶 A 底物合成 Synthesis of malonyl-CoA substrate | [ |
| 拟南芥Arabidopsis thaliana | KCS3 | 负调控蜡质合成 Negative regulation of wax biosynthesis | [ |
| 番茄Solanum lycopersicum | CER1-1 | 烷烃类合成Biosynthesis of alkanes | [ |
| 拟南芥Arabidopsis thaliana | KCS1 | 延伸C26~C30脂肪酸 Extension of C26 ~ C30 fatty acids | [ |
| 拟南芥Arabidopsis thaliana | CUT1, CER6, KCS5, KCS6 | 调控超长链脂肪酸合成 Regulation of VLCFA biosynthesis | [ |
| 拟南芥Arabidopsis thaliana | FATA/FATB | 为蜡质合成提供饱和脂肪酸 Provide saturated fatty acids for wax biosynthesis | [ |
| 拟南芥Arabidopsis thaliana | CER10 | 超长链脂肪酸合成VLCFA biosynthesis | [ |
| 拟南芥Arabidopsis thaliana | PAS2/HCD | 超长链脂肪酸合成VLCFA biosynthesis | [ |
| 拟南芥Arabidopsis thaliana | KCS20, KCS2 | 超长链脂肪酸扩展至C22 Extende VLCFAs to C22 | [ |
| 拟南芥Arabidopsis thaliana | LACS1/CER8,LCAS2, LCAS4 | 超长链脂肪酸C20~C30 合成 Synthetase activity for VLCFAs C20-C30 | [ |
| 拟南芥Arabidopsis thaliana | CER2, CER2-Like1/2 | 大于C28脂肪酸的延伸 Extension of fatty acids beyond C28 | [ |
| 拟南芥Arabidopsis thaliana | KCS9 | 延伸C22~C24脂肪酸 Extension of C22~C24 fatty acids | [ |
| 拟南芥Arabidopsis thaliana | CER1, CER22 | 烷烃类合成Biosynthesis of alkanes | [ |
| 拟南芥Arabidopsis thaliana | RST1 | 促进酰基辅酶A还原成醛类May act in reduction of acyl-CoAs to aldehydes | [ |
拟南芥 Arabidopsis thaliana | CYP96A15/MAH1 | 次级醇和酮形成Formation of secondary alcohols and ketones | [ |
| 拟南芥Arabidopsis thaliana | WSD1 | 蜡酯合成Wax ester biosynthesis | [ |
| 拟南芥Arabidopsis thaliana | GLN1, ECH | 囊泡运输Vesicle trafficking | [ |
| 拟南芥Arabidopsis thaliana | LTPG1, LTPG2 | 蜡质转运Cuticular waxs transport | [ |
| 水稻Oryza sativa | KCR1 | 超长链脂肪酸合成VLCFA biosynthesis | [ |
| 油菜Brassica napus | CER4/FAR3 | 初级醇生成Formation of primary alcohols | [ |
表1 模式植物蜡质生物合成与转运过程中的酶基因和转运体基因
Table 1 Enzyme genes and transporter genes involved in wax biosynthesis and transport in model plants
物种 Species | 基因名 Gene name | 功能 Function | 参考文献 Reference |
|---|---|---|---|
| 拟南芥Arabidopsis thaliana | CER17 | 超长链酰基辅酶A的n-6去饱和 n-6 desaturation of very long chain acyl-CoAs | [ |
| 拟南芥Arabidopsis thaliana | LACS1, LACS2, LACS4 | 蜡质合成过程中重叠 Overlap in wax synthesis | [ |
| 拟南芥Arabidopsis thaliana | Cytb5R | CER1的辅助因子Cofactor of CER1 | [ |
| 拟南芥Arabidopsis thaliana | WAX2/CER3 | 醛类合成Synthesis of aldehydes | [ |
| 拟南芥Arabidopsis thaliana | ABCG11/WBC11,ABCG12/CER5 | 蜡质转运Cuticular wax transport | [ |
| 拟南芥Arabidopsis thaliana | ACC1 | 丙二酰辅酶 A 底物合成 Synthesis of malonyl-CoA substrate | [ |
| 拟南芥Arabidopsis thaliana | KCS3 | 负调控蜡质合成 Negative regulation of wax biosynthesis | [ |
| 番茄Solanum lycopersicum | CER1-1 | 烷烃类合成Biosynthesis of alkanes | [ |
| 拟南芥Arabidopsis thaliana | KCS1 | 延伸C26~C30脂肪酸 Extension of C26 ~ C30 fatty acids | [ |
| 拟南芥Arabidopsis thaliana | CUT1, CER6, KCS5, KCS6 | 调控超长链脂肪酸合成 Regulation of VLCFA biosynthesis | [ |
| 拟南芥Arabidopsis thaliana | FATA/FATB | 为蜡质合成提供饱和脂肪酸 Provide saturated fatty acids for wax biosynthesis | [ |
| 拟南芥Arabidopsis thaliana | CER10 | 超长链脂肪酸合成VLCFA biosynthesis | [ |
| 拟南芥Arabidopsis thaliana | PAS2/HCD | 超长链脂肪酸合成VLCFA biosynthesis | [ |
| 拟南芥Arabidopsis thaliana | KCS20, KCS2 | 超长链脂肪酸扩展至C22 Extende VLCFAs to C22 | [ |
| 拟南芥Arabidopsis thaliana | LACS1/CER8,LCAS2, LCAS4 | 超长链脂肪酸C20~C30 合成 Synthetase activity for VLCFAs C20-C30 | [ |
| 拟南芥Arabidopsis thaliana | CER2, CER2-Like1/2 | 大于C28脂肪酸的延伸 Extension of fatty acids beyond C28 | [ |
| 拟南芥Arabidopsis thaliana | KCS9 | 延伸C22~C24脂肪酸 Extension of C22~C24 fatty acids | [ |
| 拟南芥Arabidopsis thaliana | CER1, CER22 | 烷烃类合成Biosynthesis of alkanes | [ |
| 拟南芥Arabidopsis thaliana | RST1 | 促进酰基辅酶A还原成醛类May act in reduction of acyl-CoAs to aldehydes | [ |
拟南芥 Arabidopsis thaliana | CYP96A15/MAH1 | 次级醇和酮形成Formation of secondary alcohols and ketones | [ |
| 拟南芥Arabidopsis thaliana | WSD1 | 蜡酯合成Wax ester biosynthesis | [ |
| 拟南芥Arabidopsis thaliana | GLN1, ECH | 囊泡运输Vesicle trafficking | [ |
| 拟南芥Arabidopsis thaliana | LTPG1, LTPG2 | 蜡质转运Cuticular waxs transport | [ |
| 水稻Oryza sativa | KCR1 | 超长链脂肪酸合成VLCFA biosynthesis | [ |
| 油菜Brassica napus | CER4/FAR3 | 初级醇生成Formation of primary alcohols | [ |

图3 植物表皮蜡质生物合成转运的转录调控机制[4]注:尖、倒T形箭头分别表示正、负调控;实线、虚线箭头分别表示直接、间接影响。
Fig. 3 Transcriptional regulatory mechanisms of plant cuticular wax biosynthesis and transport[4]Note:Sharp and inverted T-shaped arrows indicate positive and negative regulations, respectively. Solid and dashed line indicate direct and indirect impacts, respectively.
物种 Species | 转录因子TF | 受影响成分 Affected component | 参考文献 Reference | ||
|---|---|---|---|---|---|
名称 Name | 类型 Type | 靶基因 Target gene | |||
| 油菜Brassica napus | WIN1/SHN1 | AP2/EREBP | BCCP1, GPAT9, LPAT5, DGAT2, LACS2, KCS1, KCR1, CER1 | C29/C31烷烃、C28/C29醇 C29/C31 alkanes, C28/C29 alcohols | [ |
| 小麦Triticum aestivum | MYB96-2D/5D | R2R3-MYB | CER1-6A | C27/C29/C31/C33烷烃 C27/C29/C31/C33 alkanes | [ |
| 拟南芥Arabidopsis thaliana | RAP2.4 | AP2/DREB | KCS2, CER1 | C27/C29/C31/C33烷烃 C27/C29/C31/C33 alkanes | [ |
| 拟南芥Arabidopsis thaliana | WRI1/3/4 | AP2/ERF | PKp2, MAT, KASI, ENR1, FATA, BCCP2 | 二羧酸、ω-羟基脂肪酸、脂肪酸 Dicarboxylic acids, ω-hydroxy fatty acids, fatty acids | [ |
| 油菜Brassica napus | DEWAX1 | AP1\\ERF | CER1-2, CER1 | C29/C31/C33烷烃、C26脂肪酸 C29/C31/C33 alkanes, C26 fatty acid | [ |
| 拟南芥Arabidopsis thaliana | DEWAX2 | AP2/ERF | CER1, ACLA2, LACS1, LACS2, KCS12 | C29/C31烷烃、C28初级醇、C29酮 C29/C31 alkanes, C28 primary alcohol, C29 ketone | [ |
| 苹果Malus domestica | MYB30 | R2R3-MYB | WRI1, WIN1, ACBP1, LACS2, SHINE2, SHINE3, KCS1 | C29烷烃、C31醇、C29醛、C16脂肪酸、C29酮、C29/C30酯 C29 alkane, C31 alcohol, C29 aldehyde, C16 fatty acid, C29 ketone, C29/C30 esters | [ |
| 拟南芥Arabidopsis thaliana | MYB94, MYB96 | R2R3-MYB | KCR1, KCS1, KCS2/DAISY, KCS6, CER2, CER1, CER3, WSD1 | — | [ |
| 拟南芥Arabidopsis thaliana | MYB49 | R2R3-MYB | MYB41, ABCG6, KCS | 蜡质 Cuticular wax | [ |
| 拟南芥Arabidopsis thaliana | SPL9, SPL13 | SBP-box | CER1, CER4 | 烷烃类、初级醇 Alkanes, primary alcohols | [ |
| 玉米Zea mays | OCL1, HDG1 | HD-Zip Ⅳ | LTP, CYP78A6-like, ABCG, SEC14 | C25烷烃、C24/C26/C28醇、C48酯、C28/C30醛 C25 alkane, C24/C26/C28 alcohols, C48 ester, C28/C30 aldehydes | [ |
| 拟南芥Arabidopsis thaliana | MYB41 | R2R3-MYB | — | C22/C24脂肪醇、游离脂肪酸C22/C24 fatty alcohols, free fatty acids | [ |
| 拟南芥Arabidopsis thaliana | MYB96 | R2R3-MYB | RD22, GH3, KCR1, SER1, KCS1, KCS2, KCS6, PAS2, CER3, ESR, WBC11, LTP | — | [ |
| 拟南芥Arabidopsis thaliana | MYB106,MYB16 | R2R3-MYB | WIN1/SHN1, FDH, LACS2, CYP84A4, CYP77A6, KCS1, CER1, CER2, LCR, LACS2 | — | [ |
| 水稻Oryza sativa | CFL1 | ADF/cofilin | WIN1/SHN1, BDG, FDH | — | [ |
表2 调控植物表皮蜡质生物合成的转录因子
Table 2 Identified TFs involved in regulating cuticular wax biosynthesis in plants
物种 Species | 转录因子TF | 受影响成分 Affected component | 参考文献 Reference | ||
|---|---|---|---|---|---|
名称 Name | 类型 Type | 靶基因 Target gene | |||
| 油菜Brassica napus | WIN1/SHN1 | AP2/EREBP | BCCP1, GPAT9, LPAT5, DGAT2, LACS2, KCS1, KCR1, CER1 | C29/C31烷烃、C28/C29醇 C29/C31 alkanes, C28/C29 alcohols | [ |
| 小麦Triticum aestivum | MYB96-2D/5D | R2R3-MYB | CER1-6A | C27/C29/C31/C33烷烃 C27/C29/C31/C33 alkanes | [ |
| 拟南芥Arabidopsis thaliana | RAP2.4 | AP2/DREB | KCS2, CER1 | C27/C29/C31/C33烷烃 C27/C29/C31/C33 alkanes | [ |
| 拟南芥Arabidopsis thaliana | WRI1/3/4 | AP2/ERF | PKp2, MAT, KASI, ENR1, FATA, BCCP2 | 二羧酸、ω-羟基脂肪酸、脂肪酸 Dicarboxylic acids, ω-hydroxy fatty acids, fatty acids | [ |
| 油菜Brassica napus | DEWAX1 | AP1\\ERF | CER1-2, CER1 | C29/C31/C33烷烃、C26脂肪酸 C29/C31/C33 alkanes, C26 fatty acid | [ |
| 拟南芥Arabidopsis thaliana | DEWAX2 | AP2/ERF | CER1, ACLA2, LACS1, LACS2, KCS12 | C29/C31烷烃、C28初级醇、C29酮 C29/C31 alkanes, C28 primary alcohol, C29 ketone | [ |
| 苹果Malus domestica | MYB30 | R2R3-MYB | WRI1, WIN1, ACBP1, LACS2, SHINE2, SHINE3, KCS1 | C29烷烃、C31醇、C29醛、C16脂肪酸、C29酮、C29/C30酯 C29 alkane, C31 alcohol, C29 aldehyde, C16 fatty acid, C29 ketone, C29/C30 esters | [ |
| 拟南芥Arabidopsis thaliana | MYB94, MYB96 | R2R3-MYB | KCR1, KCS1, KCS2/DAISY, KCS6, CER2, CER1, CER3, WSD1 | — | [ |
| 拟南芥Arabidopsis thaliana | MYB49 | R2R3-MYB | MYB41, ABCG6, KCS | 蜡质 Cuticular wax | [ |
| 拟南芥Arabidopsis thaliana | SPL9, SPL13 | SBP-box | CER1, CER4 | 烷烃类、初级醇 Alkanes, primary alcohols | [ |
| 玉米Zea mays | OCL1, HDG1 | HD-Zip Ⅳ | LTP, CYP78A6-like, ABCG, SEC14 | C25烷烃、C24/C26/C28醇、C48酯、C28/C30醛 C25 alkane, C24/C26/C28 alcohols, C48 ester, C28/C30 aldehydes | [ |
| 拟南芥Arabidopsis thaliana | MYB41 | R2R3-MYB | — | C22/C24脂肪醇、游离脂肪酸C22/C24 fatty alcohols, free fatty acids | [ |
| 拟南芥Arabidopsis thaliana | MYB96 | R2R3-MYB | RD22, GH3, KCR1, SER1, KCS1, KCS2, KCS6, PAS2, CER3, ESR, WBC11, LTP | — | [ |
| 拟南芥Arabidopsis thaliana | MYB106,MYB16 | R2R3-MYB | WIN1/SHN1, FDH, LACS2, CYP84A4, CYP77A6, KCS1, CER1, CER2, LCR, LACS2 | — | [ |
| 水稻Oryza sativa | CFL1 | ADF/cofilin | WIN1/SHN1, BDG, FDH | — | [ |
物种 Species | 转录因子 TF | 受影响成分 Affected component | 参考文献 Reference | ||
|---|---|---|---|---|---|
名称 Name | 类型 Type | 靶基因 Target gene | |||
| 水稻Oryza sativa | WR1 | ERF | KCS2, LACS1, CER3, CUT1, FDH1/2, KCS1, LACS1-2, CER1/2, FAE1-L | C16/C20/C24/C26/C30/C32 脂肪酸、C22/C32醇、C25/C27/C29/C31烷烃、C48酯C16/C20/C24/C26/C30/C32 fatty acids, C22/C32 alcohols, C25/C27/C29/C31 alkanes, C48 ester | [ |
| 水稻Oryza sativa | MYB60 | R2R3-MYB | CER1 | C29烷烃 C29 alkane | [ |
| 小麦Triticum aestivum | WXPL2B | AP2/ERF | KCS1 | 蜡质 Cuticular wax | [ |
| 小麦Triticum aestivum | WXPL1D | AP2/ERF | KCS1, AAT1 | 蜡质 Cuticular wax | [ |
| 小麦Triticum aestivum | MYB30 | R2R3-MYB | KCS1, ECR | 超长链脂肪酸、醇、醛、烷烃、酯 VLCFAs, alcohols, aldehydes, alkanes, esters | [ |
| 大麦Hordeum vulgare | WIN1 | AP2/EREBP | KAS2, CYP86A2, CYP89A2, LACS2 | — | [ |
| 苹果Malus domestica | SHINE2 | AP2/EREBP | MYB30, MYB96, LACS2, CER1, CER3, CER6, KCS1, WIN1, DEWAX, SHINE3 | 烷烃、醇、醛、脂肪酸 Alkanes, alcohols, aldehydes, fatty acids | [ |
| 番茄Solanum lycopersicum | SHN1 | ERF | — | — | [ |
| 菜薹Brassica rapa | SHINE3 | AP2 | CER2, CYP86A7, KCS2, LACS2, CER4, KCS1, CER1 | 脂肪酸、烷烃、醇、蜡酯 Fatty acids, alkanes, alcohols, wax esters | [ |
| 黄瓜Cucumis sativus | WIN1 | AP2/ERF | CER1, CER1-1, CER4, KCS1, ABC | 蜡酯、C29/C31烷烃 Wax esters, C29/C31 alkanes | [ |
| 烟草Nicotiana tabacum | MYB12a | R2R3-MYB | LOX6, LOX5, SFAR4, GDSL2 | 脂肪酸 Fatty acids | [ |
| 脐橙Citrus sinensis | MYB30 | R2R3-MYB | CER1, CER4, MAH1, LTP3, WR4, MYB106 | 超长链脂肪酸、醇、醛、烷烃、酮 VLCFAs, alcohols, aldehydes, alkanes, ketones | [ |
| 洋桔梗Eustoma grandiflorum | MIXTA1 | R2R3-MYB | CER3, CER6, CER10, KCS1, KCR1, CYP77A6, WIN1 | 蜡质 Cuticular wax | [ |
表2 调控植物表皮蜡质生物合成的转录因子 (续表Continued)
Table 2 Identified TFs involved in regulating cuticular wax biosynthesis in plants
物种 Species | 转录因子 TF | 受影响成分 Affected component | 参考文献 Reference | ||
|---|---|---|---|---|---|
名称 Name | 类型 Type | 靶基因 Target gene | |||
| 水稻Oryza sativa | WR1 | ERF | KCS2, LACS1, CER3, CUT1, FDH1/2, KCS1, LACS1-2, CER1/2, FAE1-L | C16/C20/C24/C26/C30/C32 脂肪酸、C22/C32醇、C25/C27/C29/C31烷烃、C48酯C16/C20/C24/C26/C30/C32 fatty acids, C22/C32 alcohols, C25/C27/C29/C31 alkanes, C48 ester | [ |
| 水稻Oryza sativa | MYB60 | R2R3-MYB | CER1 | C29烷烃 C29 alkane | [ |
| 小麦Triticum aestivum | WXPL2B | AP2/ERF | KCS1 | 蜡质 Cuticular wax | [ |
| 小麦Triticum aestivum | WXPL1D | AP2/ERF | KCS1, AAT1 | 蜡质 Cuticular wax | [ |
| 小麦Triticum aestivum | MYB30 | R2R3-MYB | KCS1, ECR | 超长链脂肪酸、醇、醛、烷烃、酯 VLCFAs, alcohols, aldehydes, alkanes, esters | [ |
| 大麦Hordeum vulgare | WIN1 | AP2/EREBP | KAS2, CYP86A2, CYP89A2, LACS2 | — | [ |
| 苹果Malus domestica | SHINE2 | AP2/EREBP | MYB30, MYB96, LACS2, CER1, CER3, CER6, KCS1, WIN1, DEWAX, SHINE3 | 烷烃、醇、醛、脂肪酸 Alkanes, alcohols, aldehydes, fatty acids | [ |
| 番茄Solanum lycopersicum | SHN1 | ERF | — | — | [ |
| 菜薹Brassica rapa | SHINE3 | AP2 | CER2, CYP86A7, KCS2, LACS2, CER4, KCS1, CER1 | 脂肪酸、烷烃、醇、蜡酯 Fatty acids, alkanes, alcohols, wax esters | [ |
| 黄瓜Cucumis sativus | WIN1 | AP2/ERF | CER1, CER1-1, CER4, KCS1, ABC | 蜡酯、C29/C31烷烃 Wax esters, C29/C31 alkanes | [ |
| 烟草Nicotiana tabacum | MYB12a | R2R3-MYB | LOX6, LOX5, SFAR4, GDSL2 | 脂肪酸 Fatty acids | [ |
| 脐橙Citrus sinensis | MYB30 | R2R3-MYB | CER1, CER4, MAH1, LTP3, WR4, MYB106 | 超长链脂肪酸、醇、醛、烷烃、酮 VLCFAs, alcohols, aldehydes, alkanes, ketones | [ |
| 洋桔梗Eustoma grandiflorum | MIXTA1 | R2R3-MYB | CER3, CER6, CER10, KCS1, KCR1, CYP77A6, WIN1 | 蜡质 Cuticular wax | [ |
| [1] | ARYA G C, SARKAR S, MANASHEROVA E, et al.. The plant cuticle:an ancient guardian barrier set against long-standing rivals [J/OL].Front.Plant Sci., 2021,12:663165 [2024-03-11].. |
| [2] | LEWANDOWSKA M, KEYL A, FEUSSNER I. Wax biosynthesis in response to danger:its regulation upon abiotic and biotic stress [J]. New Phytol., 2020,227(3):698-713. |
| [3] | LIU N, CHEN J, WANG T H, et al.. Overexpression of WAX INDUCER1/SHINE1 gene enhances wax accumulation under osmotic stress and oil synthesis in Brassica napus [J/OL]. Int. J. Mol. Sci., 2019,20(18):4435 [2024-03-11]. . |
| [4] | LEE S B, SUH M C. Regulatory mechanisms underlying cuticular wax biosynthesis [J]. J. Exp. Bot., 2022,73(9):2799-2816. |
| [5] | LEE S H, FU F Y, XU S M, et al.. Global regulation of plant immunity by histone lysine methyl transferases [J].Plant Cell,2016,28(7):1640-1661. |
| [6] | LI R J, LI L M, LIU X L, et al.. Diurnal regulation of plant epidermal wax synthesis through antagonistic roles of the transcription factors SPL9 and DEWAX [J]. Plant Cell, 2019,31(11): 2711-2733. |
| [7] | LIU Q, HUANG H D, CHEN Y Q, et al.. Two Arabidopsis MYB-SHAQKYF transcription repressors regulate leaf wax biosynthesis via transcriptional suppression on DEWAX [J].New Phytol., 2022,236(6):2115-2130. |
| [8] | MÉNARD R, VERDIER G, ORS M, et al.. Histone H2B monoubiquitination is involved in the regulation of cutin and wax composition in Arabidopsis thaliana [J]. Plant Cell Physiol., 2014,55(2): 455-466. |
| [9] | YEATS T H, ROSE J K C. The formation and function of plant cuticles [J]. Plant Physiol., 2013,163(1):5-20. |
| [10] | HUANG H, BURGHARDT M, SCHUSTER A C, et al.. Chemical composition and water permeability of fruit and leaf cuticles of Olea europaea L. [J]. J. Agric. Food Chem., 2017,65(40):8790-8797. |
| [11] | BARRAJ BARRAJ R, SEGADO P, MORENO-GONZÁLEZ R, et al.. Genome-wide QTL analysis of tomato fruit cuticle deposition and composition [J/OL]. Hortic. Res., 2021,8:113 [2024-03-11]. . |
| [12] | WANG X, KONG L, ZHI P, et al.. Update on cuticular wax biosynthesis and its roles in plant disease resistance [J/OL]. Int. J. Mol. Sci., 2020,21(15):5514 [2024-03-11]. . |
| [13] | LI H, GUO Y L, CUI Q, et al.. Alkanes (C29 and C31)-mediated intracuticular wax accumulation contributes to melatonin-and ABA-induced drought tolerance in watermelon [J]. J. Plant Growth Regul., 2020, 39: 1441-1450. |
| [14] | ZHU J Y, HUANG K L, CHENG D J, et al.. Characterization of cuticular wax in tea plant and its modification in response to low temperature [J]. J. Agric. Food Chem., 2022,70(43):13849-13861. |
| [15] | SUN Q Y, CHEN W L, GE C M, et al.. Correlation of tea green leafhopper occurrence with leaf structure and biochemical components in different tea cultivars [J]. Int. J. Pest Manage., 2020, 69(1): 1-11. |
| [16] | BUENO A, ALFARHAN A, ARAND K, et al.. Effects of temperature on the cuticular transpiration barrier of two desert plants with water-spender and water-saver strategies [J]. J. Exp. Bot., 2019,70(5):1613-1625. |
| [17] | HEIL C S, WEHRHEIM S S, PAITHANKAR K S, et al.. Fatty acid biosynthesis:chain-length regulation and control [J].Chembiochem, 2019,20(18):2298-2321. |
| [18] | LI N, GÜGEL I L, GIAVALISCO P, et al.. FAX1,a novel membrane protein mediating plastid fatty acid export [J/OL]. PLoS Biol., 2015,13(2):e1002053 [2024-03-11].. |
| [19] | IQBAL T, DAS D. Biochemical investigation of membrane-bound cytochrome b5 and the catalytic domain of cytochrome b5 reductase from Arabidopsis thaliana [J]. Biochemistry, 2022,61(10):909-921. |
| [20] | CHAUDHARY K, GEETA R, PANJABI P. Origin and diversification of ECERIFERUM1 (CER1) and ECERIFERUM3(CER3) genes in land plants and phylogenetic evidence that the ancestral CER1/3 gene resulted from the fusion of pre-existing domains [J/OL]. Mol. Phylogenet. Evol., 2021,159:107101 [2024-03-11]. . |
| [21] | ZHANG D, YANG H, WANG X, et al.. Cytochrome P450 family member CYP96B5 hydroxylates alkanes to primary alcohols and is involved in rice leaf cuticular wax synthesis [J]. New Phytol., 2020,225(5):2094-2107. |
| [22] | WANG P W, DUCKNEY P, GAO E L, et al.. Keep in contact:multiple roles of endoplasmic reticulum-membrane contact sites and the organelle interaction network in plants [J]. New Phytol., 2023,238(2):482-499. |
| [23] | MCFARLANE H E, SHIN J J H, BIRD D A, et al.. Arabidopsis ABCG transporters,which are required for export of diverse cuticular lipids,dimerize in different combinations [J]. Plant Cell, 2010,22(9): 3066-3075. |
| [24] | NGUYEN V N T, LEE S B, SUH M C, et al.. OsABCG9 is an important ABC transporter of cuticular wax deposition in rice [J/OL]. Front. Plant Sci., 2018,9:960 [2024-03-11]. . |
| [25] | PANIKASHVILI D, SHI J X, SCHREIBER L, et al.. The Arabidopsis ABCG13 transporter is required for flower cuticle secretion and patterning of the petal epidermis [J]. New Phytol., 2011,190(1):113-124. |
| [26] | BESSIRE M, BOREL S, FABRE G, et al.. A member of the pleiotropic drug resistance family of atp binding cassette transporters is required for the formation of a functional cuticle in Arabidopsis [J]. Plant Cell, 2011,23(5):1958-1970. |
| [27] | HUO X H, PAN A, LEI M Y, et al.. Genome-wide characterization and functional analysis of ABCG subfamily reveal its role in cutin formation in cotton [J/OL]. Int. J. Mol. Sci., 2023,24(3):2379 [2024-03-11].. |
| [28] | LEE S B, SUH M C. Disruption of glycosylphosphatidylinositol-anchored lipid transfer protein 15 affects seed coat permeability in Arabidopsis [J]. Plant J., 2018,96(6):1206-1217. |
| [29] | LYU S Y, ZHAO H Y, PARSONS E P, et al.. The glossyhead1 allele of ACC1 reveals a principal role for multidomain acetyl-coenzyme A carboxylase in the biosynthesis of cuticular waxes by Arabidopsis [J]. Plant Physiol., 2011,157(3):1079-1092. |
| [30] | HUANG H D, YANG X P, ZHENG M L, et al.. Fine-tuning β-KETOACYL-COA SYNTHASE 3 (KCS3) and KCS12 activities in Arabidopsis is essential for maintaining cuticle integrity [J]. J. Exp. Bot., 2023, 74(21): 6575-6587. |
| [31] | WANG Y F, LIU Y S, PAN X Y, et al.. A 3-ketoacyl-CoA synthase 10 (KCS10) homologue from alfalfa enhances drought tolerance by regulating cuticular wax biosynthesis [J]. J. Agric. Food Chem., 2023,71(40):14493-14504. |
| [32] | LU Y J, CHENG X Q, JIA M J, et al.. Silencing GhFAR3.1 reduces wax accumulation in cotton leaves and leads to increased susceptibility to drought stress [J/OL]. Plant Direct, 2021,5(4):e00313 [2024-03-11]. . |
| [33] | HE J J, LI C Z, HU N, et al.. ECERIFERUM1-6A is required for the synthesis of cuticular wax alkanes and promotes drought tolerance in wheat [J]. Plant Physiol., 2022,190(3):1640-1657. |
| [34] | WU H Q, LIU L, CHEN Y F, et al.. Tomato SlCE R1-1 catalyzes the synthesis of wax alkanes which increases the drought tolerance and fruit storability [J/OL]. Hortic. Res., 2022:uhac004 [2024-03-11]. . |
| [35] | WANG W J, LIU X W, GAI X S, et al.. Cucumis sativus L.WAX2 plays a pivotal role in wax biosynthesis,influencing pollen fertility and plant biotic and abiotic stress responses [J]. Plant Cell Physiol., 2015,56(7):1339-1354. |
| [36] | CHAI G Q, LI C L, XU F, et al.. Three endoplasmic reticulum-associated fatty acyl-coenzyme a reductases were involved in the production of primary alcohols in hexaploid wheat (Triticum aestivum L.) [J/OL]. BMC Plant Biol., 2018,18(1):41 [2024-03-11]. . |
| [37] | JIANG Y Y, PENG Y T, HOU GY, et al.. A high epicuticular wax strawberry mutant reveals enhanced resistance to Tetranychus urticae Koch and Botrytis cinerea [J/OL]. Sci. Hortic., 2024,324:112636 [2024-03-11]. . |
| [38] | 韩文博.茶树CsCER1基因的功能分析及其对不同胁迫的响应模式比较 [D].福州:福建农林大学, 2022. |
| HAN W B. Functional analysis of CsCER1 gene in tea plants and comparisons of its response patterns under different stresses [D]. Fuzhou: Fujian Agriculture and Forestry University, 2022. | |
| [39] | XUE Y, XIAO S, KIM J, et al.. Arabidopsis membrane-associated acyl-CoA-binding protein ACBP1 is involved in stem cuticle formation [J]. J. Exp. Bot., 2014,65(18):5473-5483. |
| [40] | CHEN N M, SONG B, TANG S, et al.. Overexpression of the ABC transporter gene TsABCG11 increases cuticle lipids and abiotic stress tolerance in Arabidopsis [J]. Plant Biotechnol. Rep., 2018,12(5): 303-313. |
| [41] | KIM H, LEE S B, KIM H J, et al.. Characterization of glycosylphosphatidylinositol-anchored lipid transfer protein 2 (LTPG2) and overlapping function between LTPG/LTPG1 and LTPG2 in cuticular wax export or accumulation in Arabidopsis thaliana [J]. Plant Cell Physiol., 2012,53(8):1391-1403. |
| [42] | SUN W, LI Y, ZHAO Y X, et al.. The TsnsLTP4,a nonspecific lipid transfer protein involved in wax deposition and stress tolerance [J]. Plant Mol. Biol. Rep., 2015,33(4):962-974. |
| [43] | CHEN L, HU W, MISHRA N, et al.. AKR2A interacts with KCS1 to improve VLCFAs contents and chilling tolerance of Arabidopsis thaliana [J]. Plant J., 2020,103(4):1575-1589. |
| [44] | SINGH S, GEETA R, DAS S. Comparative sequence analysis across Brassicaceae,regulatory diversity in KCS5 and KCS6 homologs from Arabidopsis thaliana and Brassica juncea, and intronic fragment as a negative transcriptional regulator [J/OL]. Gene Expr. Patterns, 2020,38:119146 [2024-03-11]. . |
| [45] | SAMPANGI-RAMAIAH M H, RAVISHANKAR K V, SEETHARAMAIAH S K,et al.. Barrier against water loss:relationship between epicuticular wax composition,gene expression and leaf water retention capacity in banana [J]. Funct. Plant Biol., 2016,43(6):492-501. |
| [46] | FUKUDA N, OSHIMA Y, ARIGA H, et al.. ECERIFERUM 10 encoding an enoyl-CoA reductase plays a crucial role in osmotolerance and cuticular wax loading in Arabidopsis [J/OL].Front. Plant Sci., 2022,13:898317 [2024-03-11]. . |
| [47] | ZHU X Y, TELLIER F, GU Y, et al.. Disruption of very-long-chain-fatty acid synthesis has an impact on the dynamics of cellulose synthase in Arabidopsis thaliana [J/OL].Plants (Basel), 2020,9(11):1599 [2024-03-11]. . |
| [48] | LEE S B, JUNG S J, GO Y S, et al.. Two Arabidopsis 3-ketoacyl CoA synthase genes,KCS20 and KCS2/DAISY are functionally redundant in cuticular wax and root suberin biosynthesis,but differentially controlled by osmotic stress [J]. Plant J., 2009,60(3):462-475. |
| [49] | JESSEN D, OLBRICH A, KNÜFER J, et al.. Combined activity of LACS1 and LACS4 is required for proper pollen coat formation in Arabidopsis [J]. Plant J., 2011,68(4):715-726. |
| [50] | HASLAM T M, HASLAM R, THORAVAL D, et al.. ECERIFERUM2-LIKE proteins have unique biochemical and physiological functions in very-long-chain fatty acid elongation [J]. Plant Physiol., 2015,167(3):682-692. |
| [51] | KIM J, JUNG J H, LEE S B, et al.. Arabidopsis 3-ketoacyl-coenzyme A synthase 9 is involved in the synthesis of tetracosanoic acids as precursors of cuticular waxes,suberins,sphingolipids,and phospholipids [J]. Plant Physiol., 2013,162(2):567-580. |
| [52] | SAKURADANI E, ZHAO L F, HASLAM T M, et al.. The CER22 gene required for the synthesis of cuticular wax alkanes in Arabidopsis thaliana is allelic to CER1 [J]. Planta, 2013,237(3):731-738. |
| [53] | CHEN X B, GOODWIN S M, LIU X L, et al.. Mutation of the RESURRECTION1 locus of Arabidopsis reveals an association of cuticular wax with embryo development [J]. Plant Physiol., 2005,139(2): 909-919. |
| [54] | GREER S, WEN M, BIRD D, et al.. The cytochrome P450 enzyme CYP96A15 is the midchain alkane hydroxylase responsible for formation of secondary alcohols and ketones in stem cuticular wax of Arabidopsis [J]. Plant Physiol., 2007,145(3):653-667. |
| [55] | LI F L, WU X M, LAM P, et al.. Identification of the wax ester synthase/acyl-coenzyme A:diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis [J].Plant Physiol., 2008,148(1):97-107. |
| [56] | MCFARLANE H E, WATANABE Y, YANG W L, et al.. Golgi- and trans-Golgi network-mediated vesicle trafficking is required for wax secretion from epidermal cells[J]. Plant Physiol., 2014,164(3):1250-1260. |
| [57] | WEI H, MOVAHEDI A, LIU G Y,et al..Poplar glycosylphosphatidylinositol-anchored lipid transfer proteins respond to osmotic stress by regulating fatty acid biosynthesis [J/OL]. Ind.Crops Prod., 2022,179:114683 [2024-03-11]. . |
| [58] | KOGURE K, WATANABE A, ITO Y. Interaction of ONION2 ketoacyl CoA synthase with ketoacyl CoA reductase of rice [J]. Mol. Biol. Rep., 2022,49(2):1643-1647. |
| [59] | LONG Z B, TU M X, XU Y, et al.. Genome-wide-association study and transcriptome analysis reveal the genetic basis controlling the formation of leaf wax in Brassica napus [J]. J. Exp. Bot., 2023,74(8):2726-2739. |
| [60] | MENG S, CAO Y, LI H G, et al.. PeSHN1 regulates water-use efficiency and drought tolerance by modulating wax biosynthesis in poplar [J]. Tree Physiol., 2019,39(8):1371-1386. |
| [61] | YANG S U, KIM H, KIM R J, et al.. AP2/DREB transcription factor RAP2.4 activates cuticular wax biosynthesis in Arabidopsis leaves under drought [J/OL]. Front. Plant Sci., 2020,11:895 [2024-03-11]. . |
| [62] | PARK C S, GO Y S, SUH M C. Cuticular wax biosynthesis is positively regulated by WRINKLED 4, an AP2/ERF-type transcription factor, in Arabidopsis stems [J]. Plant J., 2016, 88(2): 257-270. |
| [63] | WANG S, BAI C, LUO N, et al.. Brassica napus BnaC9.DEWAX1 negatively regulates wax biosynthesis via transcriptional suppression of BnCER1-2 [J/OL]. Int. J. Mol. Sci., 2023,24(5):4287 [2024-03-11].. |
| [64] | KIM H, GO Y S, SUH M C. DEWAX2 transcription factor negatively regulates cuticular wax biosynthesis in Arabidopsis leaves [J]. Plant Cell Physiol., 2018,59(5):966-977. |
| [65] | YANG Q, YANG X, WANG L, et al.. Two R2R3-MYB genes cooperatively control trichome development and cuticular wax biosynthesis in Prunus persica [J]. New Phytol., 2022,234(1):179-196. |
| [66] | ZHANG Y L, ZHANG C L, WANG G L, et al.. The R 2R3 transcription factor MdMYBMYB30 modulates plant resistance against pathogens by regulating cuticular wax biosynthesis [J/OL].BMC Plant Biol.,2019,19(1):362 [2024-03-11].. |
| [67] | LEE S B, KIM H U, SUH M C. MYB94 and MYB96 additively activate cuticular wax biosynthesis in Arabidopsis [J]. Plant Cell Physiol., 2016,57(11):2300-2311. |
| [68] | ZHANG P, WANG R, YANG X, et al.. The R2R3-MYB transcription factor AtMYB49 modulates salt tolerance in Arabidopsis by modulating the cuticle formation and antioxidant defence [J]. Plant Cell Environ., 2020,43(8):1925-1943. |
| [69] | YANG J, YU S, SHI G F, et al.. Comparative analysis of R2R3-MYB transcription factors in the flower of Iris laevigata identifies a novel gene regulating tobacco cold tolerance [J]. Plant Biol. (Stuttg), 2022,24(6):1066-1075. |
| [70] | HUANG H D, ZHENG M L, JENKS M A, et al.. SPL13 together with SPL9 redundantly regulates wax biosynthesis upon drought stress [J/OL]. J. Exp. Bot., 2024, 6:erae202 [2024-03-11]. . |
| [71] | JAVELLE M, VERNOUD V, DEPÈGE-FARGEIX N D, et al.. Overexpression of the epidermis-specific homeodomain-leucine zipper Ⅳ transcription factor OUTER CELL LAYER1 in maize identifies target genes involved in lipid metabolism and cuticle biosynthesis [J]. Plant Physiol., 2010,154(1):273-286. |
| [72] | KOSMA D K, MURMU J, RAZEQ F M, et al.. AtMYB41 activates ectopic suberin synthesis and assembly in multiple plant species and cell types [J]. Plant J., 2014,80(2):216-229. |
| [73] | SEO P J, LEE S B, SUH M C, et al.. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis [J]. Plant Cell,2011,23(3):1138-1152. |
| [74] | OSHIMA Y, SHIKATA M, KOYAMA T, et al.. MIXTA-like transcription factors and wax INDUCER1/SHINE1 coordinately regulate cuticle development in Arabidopsis and Torenia fournieri [J]. Plant Cell, 2013,25(5):1609-1624. |
| [75] | WU R H, LI S B, HE S, et al.. CFL1,a WW domain protein,regulates cuticle development by modulating the function of HDG1, a class Ⅳ homeodomain transcription factor, in rice and Arabidopsis [J]. Plant Cell, 2011,23(9):3392-3411. |
| [76] | WANG Y H, WAN L Y, ZHANG L X, et al.. An ethylene response factor OsWR1 responsive to drought stress transcriptionally activates wax synthesis related genes and increases wax production in rice [J]. Plant Mol. Biol., 2012,78(3):275-288. |
| [77] | JIAN L, KANG K, CHOI Y, et al.. Mutation of OsMYB60 reduces rice resilience to drought stress by attenuating cuticular wax biosynthesis [J]. Plant J., 2022,112(2):339-351. |
| [78] | BI H H, LUANG S, LI Y, et al.. Wheat drought-responsive WXPL transcription factors regulate cuticle biosynthesis genes [J]. Plant Mol. Biol., 2017,94(1):15-32. |
| [79] | LIU L, LI H Y, WANG X Y, et al.. Transcription factor TaMYB30 activates wheat wax biosynthesis [J/OL]. Int. J. Mol. Sci., 2023,24(12):10235 [2024-03-11]. . |
| [80] | KUMAR A, YOGENDRA K N, KARRE S, et al.. WAX INDUCER1 (HvWIN1) transcription factor regulates free fatty acid biosynthetic genes to reinforce cuticle to resist Fusarium head blight in barley spikelets [J].J. Exp. Bot., 2016,67(14):4127-4139. |
| [81] | ZHANG Y L, ZHANG C L, WANG G L, et al.. Apple AP2/EREBP transcription factor MdSHINE2 confers drought resistance by regulating wax biosynthesis [J]. Planta, 2019,249(5):1627-1643. |
| [82] | AL-ABDALLAT A M, AL-DEBEI H S, AYAD J Y, et al.. Over-expression of SlSHN1 gene improves drought tolerance by increasing cuticular wax accumulation in tomato [J]. Int. J. Mol. Sci., 2014,15(11):19499-19515. |
| [83] | HUO Z Y, XU Y, YUAN S, et al.. The AP2 transcription factor BrSHINE3 regulates wax accumulation in nonheading Chinese cabbage [J/OL]. Int. J. Mol. Sci., 2022,23(21):13454 [2024-03-11].. |
| [84] | ZHANG J, YANG J J, YANG Y, et al.. Transcription factor CsWIN1 regulates pericarp wax biosynthesis in cucumber grafted on pumpkin [J/OL]. Front.Plant Sci., 2019,10:1564[2024-03-11]. . |
| [85] | WANG Z, LI Z F, WANG S S, et al.. NtMYB12a acts downstream of sucrose to inhibit fatty acid accumulation by targeting lipoxygenase and SFAR genes in tobacco [J]. Plant Cell Environ., 2021,44(3):775-791. |
| [86] | WEN X F, GENG F, CHENG Y J, et al.. Ectopic expression of CsMYB30 from Citrus sinensis enhances salt and drought tolerance by regulating wax synthesis in Arabidopsis thaliana [J]. Plant Physiol. Biochem., 2021,166:777-788. |
| [87] | WANG L S, XUE W J, LI X Q, et al.. EgMIXTA1, a MYB-type transcription factor, promotes cuticular wax formation in Eustoma grandiflorum leaves [J/OL]. Front. Plant. Sci., 2020, 11: 524947 [2024-03-11]. . |
| [88] | DASZKOWSKA-GOLEC A. Degrade or silence? RNA turnover takes control of epicuticular wax synthesis [J]. Trends Plant Sci.,2020,25(10):950-952. |
| [89] | LAM P, ZHAO L F, EVELEIGH N, et al.. The exosome and trans-acting small interfering RNAs regulate cuticular wax biosynthesis during Arabidopsis inflorescence stem development [J]. Plant Physiol., 2015, 167(2): 323-336. |
| [90] | LIU T T, TANG J Q, CHEN L, et al.. Differential expression of miRNAs and their targets in wax-deficient rapeseed [J/OL]. Sci.Rep., 2019,9:12201 [2024-03-11].. |
| [91] | KUO Y W, LIN J S, LI Y C, et al.. MicroR408 regulates defense response upon wounding in sweet potato [J]. J. Exp. Bot., 2019,70(2):469-483. |
| [92] | ZHAO W L, XIAO W Y, SUN J L, et al.. An integration of microRNA and transcriptome sequencing analysis reveal regulatory roles of miRNAs in response to chilling stress in wild rice [J/OL]. Plants (Basel), 2022,11(7):977 [2024-03-11].. |
| [93] | LIU W G, TANG X, QI X H, et al.. The ubiquitin conjugating enzyme:an important ubiquitin transfer platform in ubiquitin-proteasome system [J]. Int. J. Mol. Sci., 2020, 21(8):2894 [2024-03-11]. . |
| [94] | LIU S, TONG M, ZHAO L, et al.. The ARRE RING-type E3 ubiquitin ligase negatively regulates cuticular wax biosynthesis in Arabidopsis thaliana by controlling eceriferum1 and eceriferum3 protein levels [J/OL]. Front. Plant Sci., 2021,12:752309 [2024-03-11]. . |
| [95] | LEE H G, KIM J, SUH M C, et al.. The MIEL1 E3 ubiquitin ligase negatively regulates cuticular wax biosynthesis in Arabidopsis stems [J]. Plant Cell Physiol., 2017,58(7):1249-1259. |
| [96] | KIM H, YU S I, JUNG S H, et al.. The F-box protein SAGL1 and ECERIFERUM3 regulate cuticular wax biosynthesis in response to changes in humidity in Arabidopsis [J]. Plant Cell,2019,31(9):2223-2240. |
| [97] | ZHANG Y L, TIAN Y, MAN Y Y, et al.. Apple SUMO E3 ligase MdSIZ1 regulates cuticular wax biosynthesis by SUMOylating transcription factor MdMYB30 [J]. Plant Physiol., 2023,191(3):1771-1788. |
| [98] | WANG T Y, XING J W, LIU X Y, et al.. GCN5 contributes to stem cuticular wax biosynthesis by histone acetylation of CER3 in Arabidopsis [J]. J. Exp. Bot., 2018,69(12):2911-2922. |
| [99] | WANG X Y, ZHI P F, FAN Q X, et al.. Wheat CHD3 protein TaCHR729 regulates the cuticular wax biosynthesis required for stimulating germination of Blumeria graminis f. sp. Tritici [J]. J. Exp. Bot., 2019,70(2):701-713. |
| [100] | GUO T T, WANG D F, FANG J J, et al.. Mutations in the rice OsCHR4 gene,encoding a CHD3 family chromatin remodeler,induce narrow and rolled leaves with increased cuticular wax [J/OL]. Int. J. Mol. Sci., 2019,20(10):2567 [2024-03-11].. |
| [1] | 周峻宇, 谷雨, 吴海勇, 李明德, 刘琼峰, 周旋, 董春华. 柠檬酸强化籽粒苋修复镉污染土壤效果研究[J]. 中国农业科技导报, 2025, 27(9): 215-223. |
| [2] | 贾浩, 王洪这, 孙正文, 谷淇深, 张冬梅, 王星懿, 张艳, 卢怀玉, 马峙英, 王省芬. 棉花VOZ基因家族鉴定及GhVOZ1耐盐功能研究[J]. 中国农业科技导报, 2025, 27(9): 58-68. |
| [3] | 杨令辉, 丁志伟, 龚黎, 李学俊, 董云萍, 吕振江. 咖啡生物碱类主要化合物的提取、合成代谢途径及活性研究进展[J]. 中国农业科技导报, 2025, 27(8): 132-143. |
| [4] | 丁献华, 闫双堆, 闫明. 松木木屑加压烘焙制备高品质生物焦燃料及其特性研究[J]. 中国农业科技导报, 2025, 27(7): 204-216. |
| [5] | 蒋国斌. 我国农业植物新品种保护现状与发展趋势分析[J]. 中国农业科技导报, 2025, 27(3): 1-11. |
| [6] | 马雪晴, 冀傲冉, 郑娇莉, 曹春霞, 龚艳, 黄大野, 王蓓蓓. 植物根际促生菌促生机制及其应用研究进展[J]. 中国农业科技导报, 2025, 27(2): 13-23. |
| [7] | 李诗文, 王博, 刘静, 胡晶华, 袁亚楠, 郑欣如. 高寒草原矿区生态修复过程中植物多样性及群落稳定性分析[J]. 中国农业科技导报, 2025, 27(1): 211-221. |
| [8] | 于远介, 张青青, 马建国, 江康威, 李宏. 不同植物群落土壤有机碳及其组分的差异[J]. 中国农业科技导报, 2024, 26(9): 173-182. |
| [9] | 李冰, 朱秀敏, 李代, 杜军霞. 农用植物酵素有效成分及作用机制[J]. 中国农业科技导报, 2024, 26(7): 156-165. |
| [10] | 赵鸿硕, 曹红雨, 高广磊, 孙哲, 张英, 丁国栋. 微生物诱导碳酸钙沉淀固沙对典型沙生植物叶片性状和生理特性的影响[J]. 中国农业科技导报, 2024, 26(6): 170-182. |
| [11] | 杨招娣, 郭凤根, 王仕玉, 刘正杰, 龙雯虹. 植物生长抑制剂对藜麦农艺性状和穗发芽抗性的影响[J]. 中国农业科技导报, 2024, 26(5): 44-51. |
| [12] | 陈晨, 程大伟, 李兰, 顾红, 郭西智, 李明, 陈锦永. 油菜素内酯调控植物耐盐机理研究进展[J]. 中国农业科技导报, 2024, 26(2): 1-12. |
| [13] | 郭靖捷, 任晓萌, 蒙仲举, 王涛, 祁帅, 宋佳佳, 宝孟克那顺, 韩胜利. 半干旱风沙草原区盐湖植物防护体系土壤理化性状特征[J]. 中国农业科技导报, 2024, 26(1): 182-192. |
| [14] | 周影, 李京咏, 戴林秀, 敖弟彩, 李梓逸, 杨帆, 顾军伟, 徐强, 窦志, 高辉. 稻虾共作模式下喷施褪黑素对水稻产量形成和抗倒伏特性的影响[J]. 中国农业科技导报, 2023, 25(9): 34-42. |
| [15] | 杨丽莹, 邰孟雅, 翟夜雨, 许自成, 黄五星. 硫对植物吸收积累镉的影响及其作用机制研究进展[J]. 中国农业科技导报, 2023, 25(8): 10-21. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||