




















中国农业科技导报 ›› 2022, Vol. 24 ›› Issue (8): 44-54.DOI: 10.13304/j.nykjdb.2022.0393
陆青1( ), 梁婷1,2, 王伟伟2, 汪德州2, 吴娴1,2, 王小燕1(
), 梁婷1,2, 王伟伟2, 汪德州2, 吴娴1,2, 王小燕1( ), 唐益苗2(
), 唐益苗2( )
)
收稿日期:2022-05-11
接受日期:2022-06-02
出版日期:2022-08-15
发布日期:2022-08-22
通讯作者:
王小燕,唐益苗
作者简介:陆青 E-mail:luqing41@163.com
基金资助:
Qing LU1( ), Ting LIANG1,2, Weiwei WANG2, Dezhou WANG2, Xian WU1,2, Xiaoyan WANG1(
), Ting LIANG1,2, Weiwei WANG2, Dezhou WANG2, Xian WU1,2, Xiaoyan WANG1( ), Yimiao TANG2(
), Yimiao TANG2( )
)
Received:2022-05-11
Accepted:2022-06-02
Online:2022-08-15
Published:2022-08-22
Contact:
Xiaoyan WANG,Yimiao TANG
摘要:
热激蛋白(heat shock protein,HSPs)是一类广泛存在于植物体内的应激蛋白,在植物响应逆境过程中发挥重要作用。为进一步了解小麦HSP90-1基因并探究其相关生物学功能,搜索小麦基因组序列数据库,获得了小麦A、B、D 3个基因组上的同源序列,根据其染色体位置分别命名为TaHSP90-1-A、TaHSP90-1-B和TaHSP90-1-D,通过同源克隆从12个小麦品种中得到TaHSP90-1 DNA序列。序列分析表明,TaHSP90-1-A、TaHSP90-1-B和TaHSP90-1-D分别含有2 121、2 136和2 130 bp的完整开放阅读框,分别编码707、712和710个氨基酸;其蛋白序列C端均含有1个HSP90结构域,为HSP家族HSP90亚家族成员。TaHSP90-1在不同小麦品种中存在SNP位点,分别位于TaHSP90-1-B第2个内含子区域和TaHSP90-1-D启动子区域。系统进化分析表明,TaHSP90-1-A与二粒小麦处于同一分支;TaHSP90-1-B和TaHSP90-1-D处于同一分支。顺式作用元件分析发现,TaHSP90-1-A、TaHSP90-1-B和TaHSP90-1-D启动子区域均含有干旱响应元件(MBS element)和热响应元件(HSE element)。RT-PCR结果显示,TaHSP90-1-A、TaHSP90-1-B和TaHSP90-1-D在苗期和成熟期的叶片中表达量较高。在干旱胁迫条件下,TaHSP90-1表达量在耐旱小麦品种中较高,为旱敏感小麦品种表达量的150倍;在热胁迫条件下,TaHSP90-1在热敏感与耐热小麦品种中均上调表达,其中,TaHSP90-1-A和TaHSP90-1-D在耐热小麦品种中上调表达倍数更高。
中图分类号:
陆青, 梁婷, 王伟伟, 汪德州, 吴娴, 王小燕, 唐益苗. 小麦热激蛋白基因TaHSP90-1的克隆与表达分析[J]. 中国农业科技导报, 2022, 24(8): 44-54.
Qing LU, Ting LIANG, Weiwei WANG, Dezhou WANG, Xian WU, Xiaoyan WANG, Yimiao TANG. Cloning and Expression Analysis of Wheat Heat Shock Protein Gene TaHSP90-1[J]. Journal of Agricultural Science and Technology, 2022, 24(8): 44-54.
引物名称 Primer name | 引物序列 Primer sequence (5’-3’) | 用途 Purpose | 扩增产物长度PCR product length/bp | 退火温度 Temperature/℃ |
|---|---|---|---|---|
| TaHSP90-1-A-F | TCGAGAAGTGGCAGCGGAGACGGCA | 克隆基因Gene cloning | 2 318 | 62 |
| TaHSP90-1-A-R | AGGAAATGAGACTCTTCTTCAATCT | |||
| TaHSP90-1-B-F | CCAATCTTCCGGCGAAAGAGAGGCC | 2 236 | 60 | |
| TaHSP90-1-B-R | TGAAATGAGATTCTTCAAGAGAT | |||
| TaHSP90-1-D-F | TGACAGCCATGGCGGACGTGCAG | 2 230 | 60 | |
| TaHSP90-1-D-R | GAGGTCGACTGAAGAATTTCAGT | |||
| TaHSP90-1-A-qPCR-F | GGGGGGAGAGACCACAAG | 荧光定量PCR Real-time PCR | 117 | 60 |
| TaHSP90-1-A-qPCR-R | CGGAGATGGGATCGCTAGG | |||
| TaHSP90-1-B-qPCR-F | GCAGTATGTGTGGGAGTCGC | 194 | 60 | |
| TaHSP90-1-B-qPCR-R | TCCAGAGGTAGATGGGGTAG | |||
| TaHSP90-1-D-qPCR-F | AGCAGTACGTGTGGGAGTCGC | 199 | 60 | |
| TaHSP90-1-D-qPCR-R | TCGGTCCAGAGGTAGATGGGG | |||
| TaHSP90-1-A-promoter-F | CAAGTACATGGACCATCTAGCG | 克隆启动子Cloning promoter | 2 115 | 64 |
| TaHSP90-1-A-promoter-R | GACCTTAGCAGAAATGGGGAGGC | |||
| TaHSP90-1-B-promoter-F | TAACATTGTGAAACTGATTATTT | 2 234 | 60 | |
| TaHSP90-1-B-promoter-R | AATGGACCTCAGAAGAAACGGG | |||
| TaHSP90-1-D-promoter-F | GAAAAAGATGTTGAATTTATTGT | 2 178 | 60 | |
| TaHSP90-1-D-promoter-R | AGGGAAGCGGTTCGCCTTGGCCT | |||
| Actin-F | TACTCCCTCACAACAACCG | 内参 Quantitative reference | 317 | 60 |
| Actin-R | AGAACCTCCACTGAGAACAA |
表1 引物名称及序列
Table 1 Primer name and sequence
引物名称 Primer name | 引物序列 Primer sequence (5’-3’) | 用途 Purpose | 扩增产物长度PCR product length/bp | 退火温度 Temperature/℃ |
|---|---|---|---|---|
| TaHSP90-1-A-F | TCGAGAAGTGGCAGCGGAGACGGCA | 克隆基因Gene cloning | 2 318 | 62 |
| TaHSP90-1-A-R | AGGAAATGAGACTCTTCTTCAATCT | |||
| TaHSP90-1-B-F | CCAATCTTCCGGCGAAAGAGAGGCC | 2 236 | 60 | |
| TaHSP90-1-B-R | TGAAATGAGATTCTTCAAGAGAT | |||
| TaHSP90-1-D-F | TGACAGCCATGGCGGACGTGCAG | 2 230 | 60 | |
| TaHSP90-1-D-R | GAGGTCGACTGAAGAATTTCAGT | |||
| TaHSP90-1-A-qPCR-F | GGGGGGAGAGACCACAAG | 荧光定量PCR Real-time PCR | 117 | 60 |
| TaHSP90-1-A-qPCR-R | CGGAGATGGGATCGCTAGG | |||
| TaHSP90-1-B-qPCR-F | GCAGTATGTGTGGGAGTCGC | 194 | 60 | |
| TaHSP90-1-B-qPCR-R | TCCAGAGGTAGATGGGGTAG | |||
| TaHSP90-1-D-qPCR-F | AGCAGTACGTGTGGGAGTCGC | 199 | 60 | |
| TaHSP90-1-D-qPCR-R | TCGGTCCAGAGGTAGATGGGG | |||
| TaHSP90-1-A-promoter-F | CAAGTACATGGACCATCTAGCG | 克隆启动子Cloning promoter | 2 115 | 64 |
| TaHSP90-1-A-promoter-R | GACCTTAGCAGAAATGGGGAGGC | |||
| TaHSP90-1-B-promoter-F | TAACATTGTGAAACTGATTATTT | 2 234 | 60 | |
| TaHSP90-1-B-promoter-R | AATGGACCTCAGAAGAAACGGG | |||
| TaHSP90-1-D-promoter-F | GAAAAAGATGTTGAATTTATTGT | 2 178 | 60 | |
| TaHSP90-1-D-promoter-R | AGGGAAGCGGTTCGCCTTGGCCT | |||
| Actin-F | TACTCCCTCACAACAACCG | 内参 Quantitative reference | 317 | 60 |
| Actin-R | AGAACCTCCACTGAGAACAA |
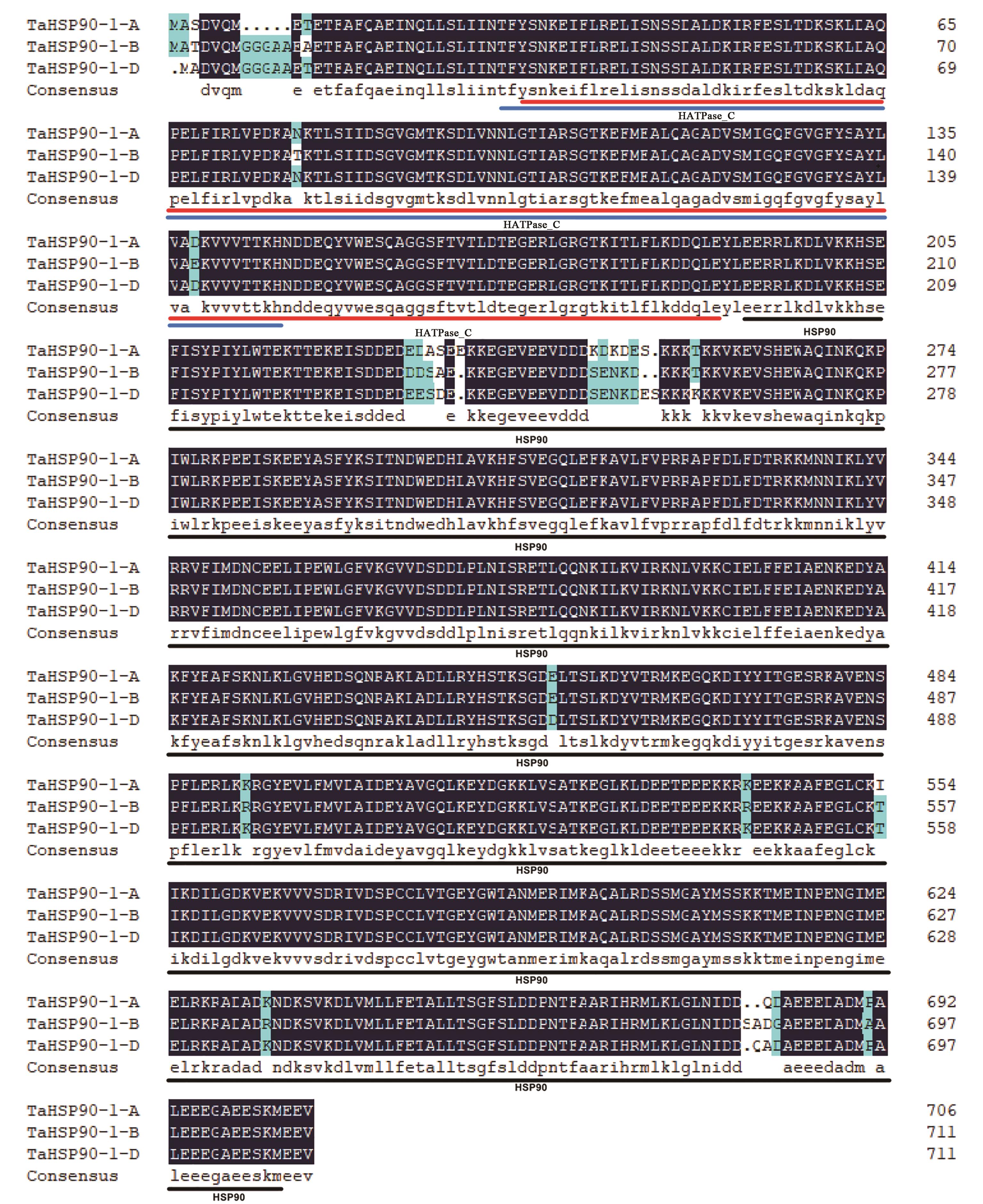
图1 TaHSP90-1同源蛋白多序列比对注:深蓝色背景的氨基酸序列表示序列完全匹配;浅蓝色背景的氨基酸序列表示氨基酸序列匹配有差异;蓝色和红色下划线表示HATPase_C结构域;黑色下划线表示HSP90结构域。
Fig. 1 Multi sequence alignment of TaHSP90-1 homologous proteinNote:The amino acid sequence on the dark blue background indicates that the sequence is completely matched; the amino acid sequence on the light blue background indicates that the amino acid sequence matching is different; the blue and red underline indicates HATPase_C domain; the black underline indicates HSP90 domain.
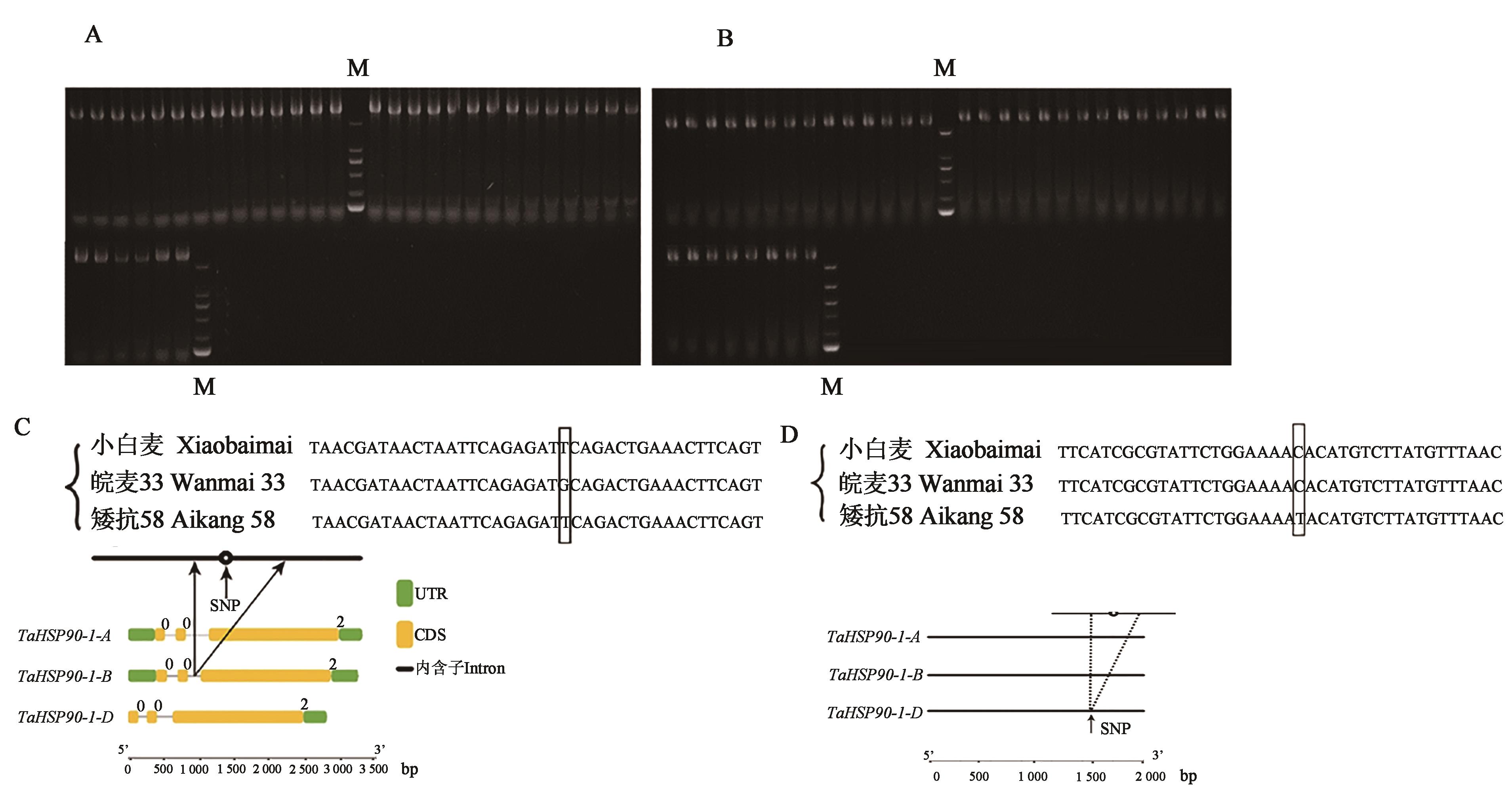
图2 小麦TaHSP90-1基因全长及启动子序列扩增结果与分析A:小麦TaHSP90-1基因全长序列扩增结果;B:小麦TaHSP90-1启动子扩增结果;C:小白麦、皖麦33、矮抗58 TaHSP90-1基因结构和序列变异;D:TaHSP90-1基因启动子的结构和序列变异。M—DL 2 000 分子量标记;UTR—非翻译区;CDS—编码序列
Fig. 2 Amplification results and analysis of the full-length sequence and promoter of wheat TaHSP90-1 geneA:Full length amplification of wheat TaHSP90-1 gene; B:Amplification results of wheat TaHSP90-1 promoter; C:Sketch map of structure and sequence variation of gene TaHSP90-1 in Xiaobaimai,Wanmai 33 and Aikang 58; D:Sketch map of structure and sequence variation of gene TaHSP90-1 promoter in Xiaobaimai,Wanmai 33 and Aikang 58. M—DL 2 000 marker; UTR—Untranslated region; CDS—Coding sequence
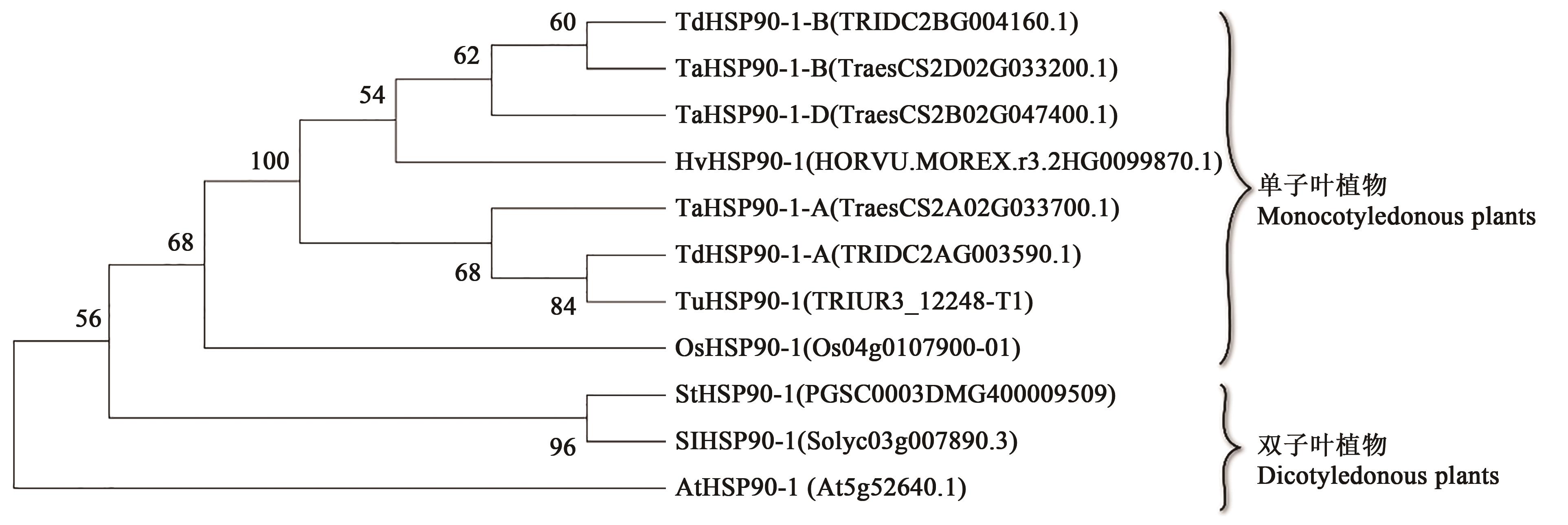
图3 小麦TaHSP90-1蛋白与其他植物的同源蛋白序列的系统进化分析注:At—拟南芥;Os—水稻; Tu—乌拉尔图小麦;Hv—大麦;Td—二粒小麦;Sl—番茄;St—马铃薯;Ta—小麦。
Fig.3 Phylogenetic analysis of wheat TaHSP90-1 protein and other plant homologous protein sequencesNote:At—Arabidopsis thaliana; Os—Oryza sativa; Tu—Triticum urartu; Hv—Hordeum vulgare; Td—Triticum dicoccoide; Sl—Solanum lycopersicum; St—Solanumtuberosum; Ta—Triticum aestivum.
基因 Gene | 顺式作用元件序列 Cis-acting element sequence (5’-3’) | 顺式作用元件 Cis-acting element | 长度 Length/bp |
|---|---|---|---|
| TaHSP90-1-A | CAACTG | MBS | 6 |
| TaHSP90-1-D | CAACTG | MBS | 6 |
| TaHSP90-1-D | CAACTG | MBS | 6 |
| TaHSP90-1-A | ACATCCTCCG | HSE | 10 |
| TaHSP90-1-A | AGATCCTCTAG | HSE | 11 |
| TaHSP90-1-A | GAATCCTTGTG | HSE | 11 |
| TaHSP90-1-A | TTTTTCCTTT | HSE | 10 |
| TaHSP90-1-A | GGCATCCTGGG | HSE | 11 |
| TaHSP90-1-B | AGATTGGAACTCCC | HSE | 14 |
| TaHSP90-1-B | CTCACGAATCGCA | HSE | 13 |
| TaHSP90-1-B | CTCACGAATCGCA | HSE | 13 |
| TaHSP90-1-D | ACGAGAAAATGCA | HSE | 13 |
| TaHSP90-1-D | ACAACTCCCCAGT | HSE | 13 |
| TaHSP90-1-D | ACAAATCCATGAAC | HSE | 14 |
| TaHSP90-1-D | TTTTGAACCGGT | HSE | 12 |
| TaHSP90-1-D | ATGGTCCGTATGT | HSE | 13 |
| TaHSP90-1-D | CAAGTAGAAGCTTCG | HSE | 15 |
表2 TaHSP90-1顺式作用元件统计
Table 2 Cis-acting element statistics of TaHSP90-1
基因 Gene | 顺式作用元件序列 Cis-acting element sequence (5’-3’) | 顺式作用元件 Cis-acting element | 长度 Length/bp |
|---|---|---|---|
| TaHSP90-1-A | CAACTG | MBS | 6 |
| TaHSP90-1-D | CAACTG | MBS | 6 |
| TaHSP90-1-D | CAACTG | MBS | 6 |
| TaHSP90-1-A | ACATCCTCCG | HSE | 10 |
| TaHSP90-1-A | AGATCCTCTAG | HSE | 11 |
| TaHSP90-1-A | GAATCCTTGTG | HSE | 11 |
| TaHSP90-1-A | TTTTTCCTTT | HSE | 10 |
| TaHSP90-1-A | GGCATCCTGGG | HSE | 11 |
| TaHSP90-1-B | AGATTGGAACTCCC | HSE | 14 |
| TaHSP90-1-B | CTCACGAATCGCA | HSE | 13 |
| TaHSP90-1-B | CTCACGAATCGCA | HSE | 13 |
| TaHSP90-1-D | ACGAGAAAATGCA | HSE | 13 |
| TaHSP90-1-D | ACAACTCCCCAGT | HSE | 13 |
| TaHSP90-1-D | ACAAATCCATGAAC | HSE | 14 |
| TaHSP90-1-D | TTTTGAACCGGT | HSE | 12 |
| TaHSP90-1-D | ATGGTCCGTATGT | HSE | 13 |
| TaHSP90-1-D | CAAGTAGAAGCTTCG | HSE | 15 |
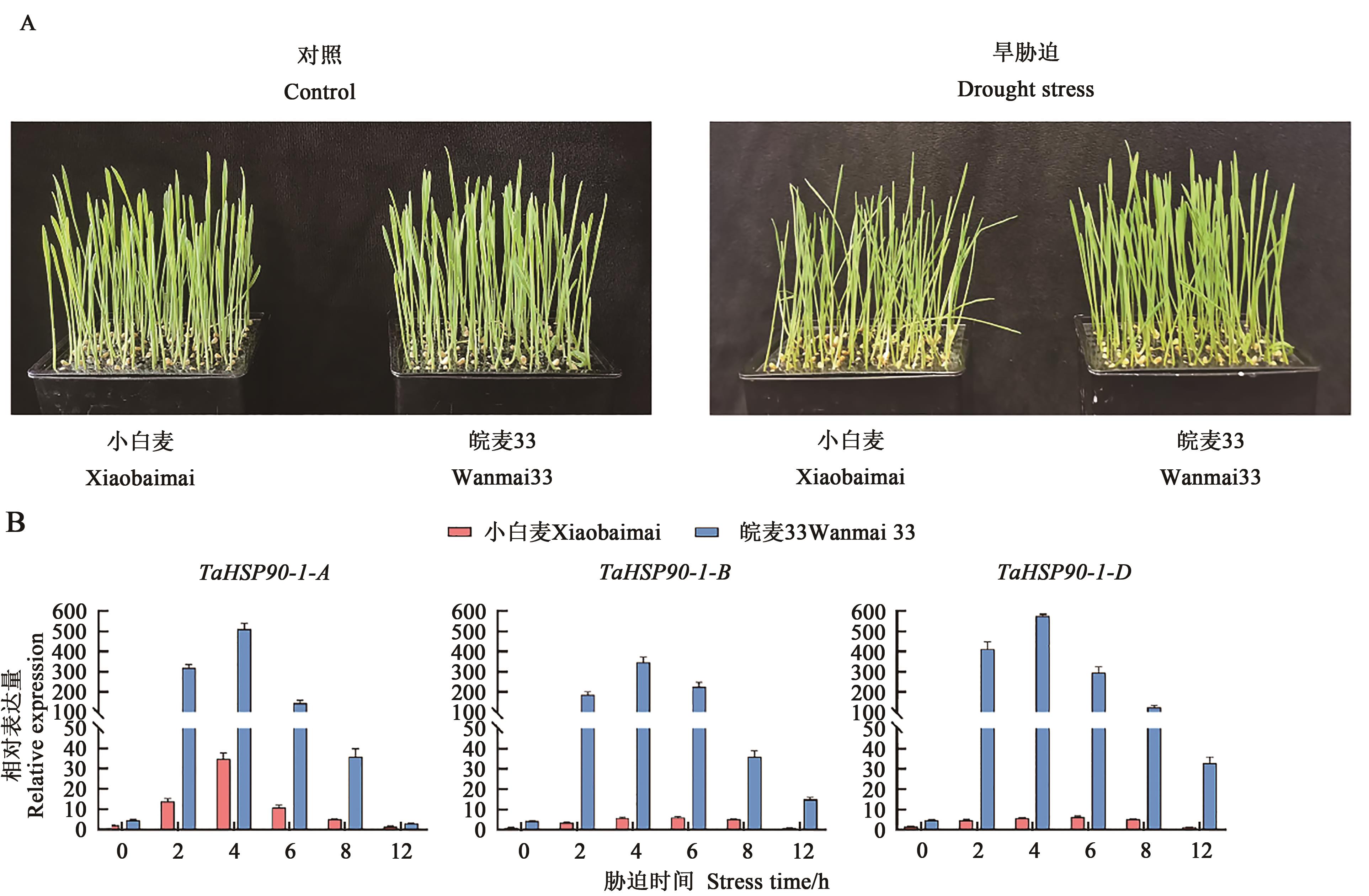
图5 干旱胁迫下小麦TaHSP90-1在不同品种中的表达分析A:旱胁迫12 h生长情况;B:旱胁迫后TaHSP90-1基因表达
Fig. 5 Expression analysis of wheat TaHSP90-1 in different varieties under drought stressA: 12 h growth of Xiaobaimai and Wanmai 33 under drought stress; B: Expression of TaHSP90-1 gene in Xiaobaimai and Wanmai 33 after drought stress
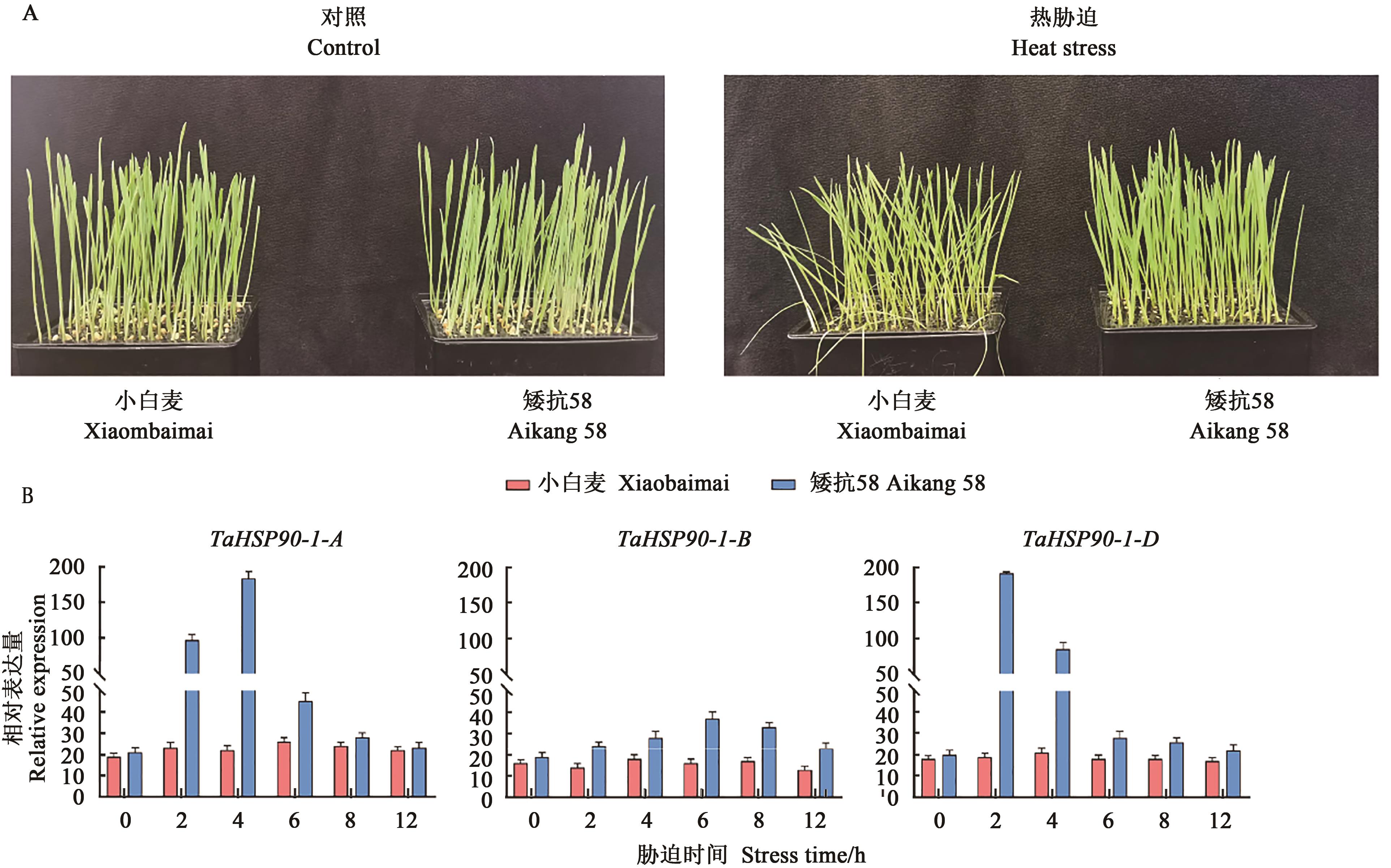
图6 热胁迫下小麦TaHSP90-1在不同品种中的表达分析A:小白麦和矮抗58热胁迫12 h后的表型;B:小白麦和矮抗58热胁迫后TaHSP90-1基因表达
Fig. 6 Expression analysis of wheat TaHSP90-1 in different varieties under heat stressA: Phenotype of Xiaobaimai and Aikang 58 after heat stress 12 h; B: Expression of TaHSP90-1 gene in Xiaobaimai and Aikang 58 after heat stress
| 1 | UL HAQ S, KHAN A, ALI M, et al.. Heat shock proteins: dynamic biomolecules to counter plant biotic and abiotic stresses [J/OL]. Int. J. Mol. Sci., 2019, 20(21):5321 [2022-03-20]. . |
| 2 | HOPPE T, COHEN E J G. Organismal protein homeostasis mechanisms [J]. Genetics, 2020, 215(4):889-901. |
| 3 | NAKAI A J N S, BIOLOGY M. Molecular basis of HSF regulation [J]. Nat. Struct. Mol. Biol., 2016, 23(2):93-95. |
| 4 | ALBERTOS P, DUNDAR G, SCHENK P, et al.. Transcription factor BES1 interacts with HSFA1 to promote heat stress resistance of plants [J/OL]. EMBO J., 2022, 41(3):8664 [2022-03-20]. . |
| 5 | WANG W X, VINOCUR B, SHOSEYOV O, et al.. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response [J]. Trends Plant Sci., 2004, 9(5):244-252. |
| 6 | 栗振义,龙瑞才,张铁军,等.植物热激蛋白研究进展[J].生物技术通报,2016,32(2):7-13. |
| LI Z Y, LONG R C, ZHANG T J, et al.. Research progress on plant heat shock protein [J]. Biotechnol. Bull., 2016, 32(2):7-13. | |
| 7 | RAMAN S, SUGUNA K. Functional characterization of heat-shock protein 90 from Oryza sativa and crystal structure of its N-terminal domain [J]. Acta Crystallogr. F., 2015, 71:688-696. |
| 8 | SONG H M, ZHAO R M, FAN P X, et al.. Overexpression of AtHsp90.2, AtHsp90.5 and AtHsp90.7 in Arabidopsis thaliana enhances plant sensitivity to salt and drought stresses [J]. Planta, 2009, 229(4):955-964. |
| 9 | SAMAKOVLI D, TICHA T, VAVRDOVA T, et al.. YODA-HSP90 module regulates phosphorylation-dependent inactivation of SPEECHLESS to control stomatal development under acute heat stress in Arabidopsis [J]. Mol. Plant, 2020, 13(4):612-633. |
| 10 | XIANG J H, CHEN X B, HU W, et al.. Overexpressing heat-shock protein OsHSP50.2 improves drought tolerance in rice [J]. Plant Cell Rep. , 2018, 37(11):1585-1595. |
| 11 | 张海.水稻HSP90基因家族功能研究[D].雅安:四川农业大学,2016. |
| ZHANG H.Function analysis of the OsHSP90 family in rice [D]. Ya'an: Sichuan Agricultural University, 2016. | |
| 12 | 刘玲玲,柳思思,翁建峰,等.玉米热激蛋白基因ZmHsp90-1的克隆及表达分析[J].作物学报,2012,38(10):1839-1846. |
| LIU L L, LIU S S, WENG J F, et al.. Cloning and expression analysis of heat shock protein gene ZmHsp90-1 in maize [J]. Acta Agron. Sin., 2012,38(10):1839-1846. | |
| 13 | 卢云泽.小麦灌浆期旗叶响应高温胁迫的蛋白组学与热响应关键基因HSP90的全基因组分析[D].杨凌:西北农林科技大学,2018. |
| LU Y Z. Proteomics analysis of flag leaves in response to heat stress and genome wide analysis of the important heat responsive gene,HSP90 during grain filling stage in wheat [D]. Yangling: Northwest A&F University, 2018. | |
| 14 | 张晓丽,代红军.植物RNA提取方法的研究进展[J].北方园艺,2014(8):175-178. |
| ZHANG X L, DAI H J. Research progress on extraction method of plant RNA [J]. Northern Hortic., 2014(8):175-178. | |
| 15 | 任毅,颜安,张芳,等.国内外301份小麦品种(系)种子萌发期抗旱性鉴定及评价[J].干旱地区农业研究,2019,37(3):1-14. |
| REN Y, YAN A, ZHANG F,et al..Identification and evaluation of drought tolerance of 301 wheat varieties (lines) at germination stage [J]. Agric. Res. Acid Areas, 2019, 37(3):1-14. | |
| 16 | LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔ CT method [J]. Methods, 2001, 25(4): 402-408. |
| 17 | LUO A, LI X B, ZHANG X C, et al.. Identification of AtHsp 90.6 involved in early embryogenesis and its structure prediction by molecular dynamics simulations [J/OL]. Roy. Soc. Open. Sci., 2019, 6(5):219 [2022-03-20]. . |
| 18 | TAGA Y, TAKAI R, KANEDA T, et al.. Role of OsHSP90 and IREN, Ca2+ dependent nuclease, in plant hypersensitive cell death induced by transcription factor OsNAC4 [J]. Plant Signal Behav., 2009, 4(8):740-742. |
| 19 | WANG G, FAN R, WANG X, et al.. TaRAR1 and TaSGT1 associate with TaHsp90 to function in bread wheat (Triticum aestivum L.) seedling growth and stripe rust resistance [J]. Plant Mol. Biol., 2015, 87(6):577-589. |
| 20 | 刘云飞,周国治,万红建,等.番茄热激蛋白90的全基因组鉴定及分析[J].遗传,2014,36(10):1043-1052. |
| LIU Y F, ZHOU G Z, WAN H J, et al.. Genome-wide identification and analysis of heat shock protein 90 in tomato. [J]. Hereditas, 2014, 36(10):1043-1052. | |
| 21 | 桑璐曼,汤沙,张仁梁,等.谷子热激蛋白HSP90家族基因鉴定及分析[J/OL].植物遗传资源学报,2022:1 [2022-03-20]. . |
| SANG L M, TANG S, ZHANG R L, et al.. Identification and analysis of heat shock protein HSP90 family genes in foxtail millet [J/OL]. J. Plant Genetic Res., 2022:1 [2022-03-20]. . | |
| 22 | 杜延飞.水稻OsHSP90基因的克隆及功能初步分析[D].雅安:四川农业大学, 2015. |
| DU Y F.Cloning and functional analysis of OsHSP90 gene in Rice [D]. Ya'an: Sichuan Agricultural University, 2015. | |
| 23 | CHITTORI S, HONG J, BAI Y, et al.. Structure of the primed state of the ATPase domain of chromatin remodeling factor ISWI bound to the nucleosome [J]. Nucleic Acids Res., 2019, 47(17): 9400-9409. |
| 24 | PRODROMOU C, PEARL L H. Structure and functional relationships of Hsp90 [J]. Curr. Cancer Drug Targets, 2003, 3(5):301-323. |
| 25 | COIRO M, DOYLE J A, HILTON J. How deep is the conflict between molecular and fossil evidence on the age of angiosperms? [J]. New Phytol., 2019, 223(1):83-99. |
| 26 | SAMAKOVLI D, TICHÁ T, AMAJ J, et al.. HSP90 chaperones regulate stomatal differentiation under normal and heat stress conditions [J/OL]. Plant Signal Behav., 2020, 15(9):1789817 [2022-03-20]. . |
| 27 | GUPTA A, RICO-MEDINA A, CAO-DELGADO A I. The physiology of plant responses to drought [J]. Science, 2020, 368(6488):266-279. |
| [1] | 刘一凡, 刘少帅, 臧瑞, 李洋, 刘薇, 李婷婷, 刘旦梅, 刘登才, 李爱丽, 毛龙, 王翔, 耿帅锋. 168份小麦种质资源品质性状分析[J]. 中国农业科技导报, 2025, 27(9): 44-57. |
| [2] | 吕彩霞, 李永福, 信会男, 李娜, 赖宁, 耿庆龙, 陈署晃. 缓释氮肥对滴灌冬小麦产量及土壤硝/铵态氮的影响[J]. 中国农业科技导报, 2025, 27(8): 179-186. |
| [3] | 朱强, 车宗贤, 崔恒, 张久东, 包兴国. 绿肥替代氮肥对麦田温室气体的影响[J]. 中国农业科技导报, 2025, 27(7): 182-189. |
| [4] | 胡懿, 公杰, 赵玮, 程蓉, 柳忠玉, 高世庆, 杨亚珍. 小麦PHY基因家族鉴定及热胁迫下表达分析[J]. 中国农业科技导报, 2025, 27(7): 30-43. |
| [5] | 喻好好, 董相书, 赵颢, 李忠贤, 胡发广, 李亚男, 娄予强, 何飞飞. 干旱胁迫下小粒咖啡SNP位点与可变剪接分析[J]. 中国农业科技导报, 2025, 27(6): 72-82. |
| [6] | 呼斯乐, 包玉龙, 图布新巴雅尔null, 陶际峰, 郭恩亮. 基于无人机高光谱和集成学习的春小麦叶绿素含量反演[J]. 中国农业科技导报, 2025, 27(6): 93-103. |
| [7] | 史硕, 冯宇, 李亮, 孟瑞, 章延泽, 杨秀荣. 印度梨形孢介导小麦抗纹枯病的转录组分析及关键基因筛选[J]. 中国农业科技导报, 2025, 27(5): 133-145. |
| [8] | 马蓓, 公杰, 杜银柯, 甘雨薇, 程蓉, 朱波, 易丽霞, 马锦绣, 高世庆. 小麦花粉孔发育相关TaINP1基因鉴定及表达分析[J]. 中国农业科技导报, 2025, 27(4): 22-35. |
| [9] | 秦岭, 王艳珂, 陈二影, 杨延兵, 黎飞飞, 张梦媛, 管延安. ABA缓解谷子幼苗干旱胁迫生理特性分析[J]. 中国农业科技导报, 2025, 27(4): 36-44. |
| [10] | 陈宜新, 杨秀波, 田士军, 王聪, 白志英, 李存东, 张科. 陆地棉GhCOMT28对干旱胁迫的响应[J]. 中国农业科技导报, 2025, 27(4): 45-56. |
| [11] | 薛振宇, 张康康, 张元元, 闫强强, 姚立蓉, 张宏, 孟亚雄, 司二静, 李葆春, 马小乐, 王化俊, 汪军成. 优质抗旱小麦种质的筛选及功能基因检测[J]. 中国农业科技导报, 2025, 27(1): 35-49. |
| [12] | 高山, 闫晓翠, 王楠, 张梦洁, 李友鹏, 刁文达, 段会军. 基于10K SNP芯片分析255份玉米种质资源的遗传多样性[J]. 中国农业科技导报, 2024, 26(8): 20-33. |
| [13] | 孙宪印, 牟秋焕, 米勇, 吕广德, 亓晓蕾, 孙盈盈, 尹逊栋, 王瑞霞, 吴科, 钱兆国, 赵岩, 高明刚. 基于GT双标图对小麦新品系的分类评价[J]. 中国农业科技导报, 2024, 26(7): 14-24. |
| [14] | 桂意云, 李海碧, 梁强, 杨荣仲, 韦金菊, 韦德斌, 李文教, 刘昔辉, 周会. 基于人为控水和自然水分胁迫下的甘蔗茎节生长变化[J]. 中国农业科技导报, 2024, 26(7): 25-36. |
| [15] | 鲍新跃, 陈红敏, 王伟伟, 唐益苗, 房兆峰, 马锦绣, 汪德州, 左静红, 姚占军. 小麦TaCOBL-5基因克隆及表达分析[J]. 中国农业科技导报, 2024, 26(6): 11-21. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||