




















Journal of Agricultural Science and Technology ›› 2025, Vol. 27 ›› Issue (10): 84-94.DOI: 10.13304/j.nykjdb.2024.0747
• BIOTECHNOLOGY & LIFE SCIENCE • Previous Articles Next Articles
Hailong CHEN( ), Jialiang DAI, Jing DENG, Xinxing GAO, Guanxing ZHU, Qingming HE, Nianqing ZHU
), Jialiang DAI, Jing DENG, Xinxing GAO, Guanxing ZHU, Qingming HE, Nianqing ZHU
Received:2024-09-10
Accepted:2025-02-10
Online:2025-10-15
Published:2025-10-15
陈海龙( ), 戴佳良, 邓晶, 高新星, 朱官兴, 何清明, 朱年青
), 戴佳良, 邓晶, 高新星, 朱官兴, 何清明, 朱年青
作者简介:陈海龙E-mail: xi_zhilang@ 126.com
基金资助:CLC Number:
Hailong CHEN, Jialiang DAI, Jing DENG, Xinxing GAO, Guanxing ZHU, Qingming HE, Nianqing ZHU. Co-expression of SNF1 and MetK1 Promoted S-adenosine-L-methionine Synthesis in Saccharomyces cerevisiae[J]. Journal of Agricultural Science and Technology, 2025, 27(10): 84-94.
陈海龙, 戴佳良, 邓晶, 高新星, 朱官兴, 何清明, 朱年青. SNF1与MetK1共表达促进酵母S-腺苷-L-甲硫氨酸合成[J]. 中国农业科技导报, 2025, 27(10): 84-94.
Add to citation manager EndNote|Ris|BibTeX
URL: https://nkdb.magtechjournal.com/EN/10.13304/j.nykjdb.2024.0747
| 基因Gene | 正向引物Forward primer (5’-3’) | 反向引物Reverse primer (5’-3’) |
|---|---|---|
| HXT1 | TGTGCCATTGGTGGTATCGT | ACCACCGACACCTAAACCAG |
| HXK1 | TACTGGTGTCAACGGTGCTT | GTTCGTCGACAGCAACATCG |
| HXK2 | CTGCTCCAATGGCCATCAAC | AAGGTTTGTTGGCCTGGTCT |
| PFK1 | TGGTCTTGTCGGTTCCATCG | AAGGTTTGTTGGCCTGGTCT |
| TDH1 | TTGAGGTTGTTGCTGTCAACG | GCTTGTCGTCATGGGAAACAG |
| PGK1 | AGGCTTCTGCCCCAGGTTC | CAGCACGTTGTGGCAAGTC |
| PYK | CGACTCAGATGCTGGATTCA | CCGTTTCTCCAGAAAGCATAA |
| ALD6 | GAACTTCACCACCTTAGAGCCA | GCAGCGGGTTTCAAGATACA |
| ACS1 | GCTGCAAAGGATAAGGATGG | GCCTCAATTTCAGCGGTAGA |
| SER33 | CTCGTCGTGTAAGCATT | ACTTGTGGAACTCTACTT |
| SER1 | ACAACTCAGCCTATAATACAA | CCAATGCCTCATATAATATCTT |
| SER2 | CCTATCGTAGACGGACAG | CACCATACAACTTGCTTCA |
| MET17 | AAATGGATTGGTGGTCATGG | GAAAGAGGCAAATGGGTTCA |
| MET6 | TGGAAGCTGCCGGTATCAAG | GCAACTCTGAAAGCTTCGGC |
| SAM2 | TGAATCCGTCGGTGAAGGTC | AGCTGTTTCACAGGCAACCT |
| ACT1 | ACGCTCCTCGTGCTGTCTTC | GTTCTTCTGGGGCAACTCTCA |
Table 1 Primers used in RT-qPCR
| 基因Gene | 正向引物Forward primer (5’-3’) | 反向引物Reverse primer (5’-3’) |
|---|---|---|
| HXT1 | TGTGCCATTGGTGGTATCGT | ACCACCGACACCTAAACCAG |
| HXK1 | TACTGGTGTCAACGGTGCTT | GTTCGTCGACAGCAACATCG |
| HXK2 | CTGCTCCAATGGCCATCAAC | AAGGTTTGTTGGCCTGGTCT |
| PFK1 | TGGTCTTGTCGGTTCCATCG | AAGGTTTGTTGGCCTGGTCT |
| TDH1 | TTGAGGTTGTTGCTGTCAACG | GCTTGTCGTCATGGGAAACAG |
| PGK1 | AGGCTTCTGCCCCAGGTTC | CAGCACGTTGTGGCAAGTC |
| PYK | CGACTCAGATGCTGGATTCA | CCGTTTCTCCAGAAAGCATAA |
| ALD6 | GAACTTCACCACCTTAGAGCCA | GCAGCGGGTTTCAAGATACA |
| ACS1 | GCTGCAAAGGATAAGGATGG | GCCTCAATTTCAGCGGTAGA |
| SER33 | CTCGTCGTGTAAGCATT | ACTTGTGGAACTCTACTT |
| SER1 | ACAACTCAGCCTATAATACAA | CCAATGCCTCATATAATATCTT |
| SER2 | CCTATCGTAGACGGACAG | CACCATACAACTTGCTTCA |
| MET17 | AAATGGATTGGTGGTCATGG | GAAAGAGGCAAATGGGTTCA |
| MET6 | TGGAAGCTGCCGGTATCAAG | GCAACTCTGAAAGCTTCGGC |
| SAM2 | TGAATCCGTCGGTGAAGGTC | AGCTGTTTCACAGGCAACCT |
| ACT1 | ACGCTCCTCGTGCTGTCTTC | GTTCTTCTGGGGCAACTCTCA |
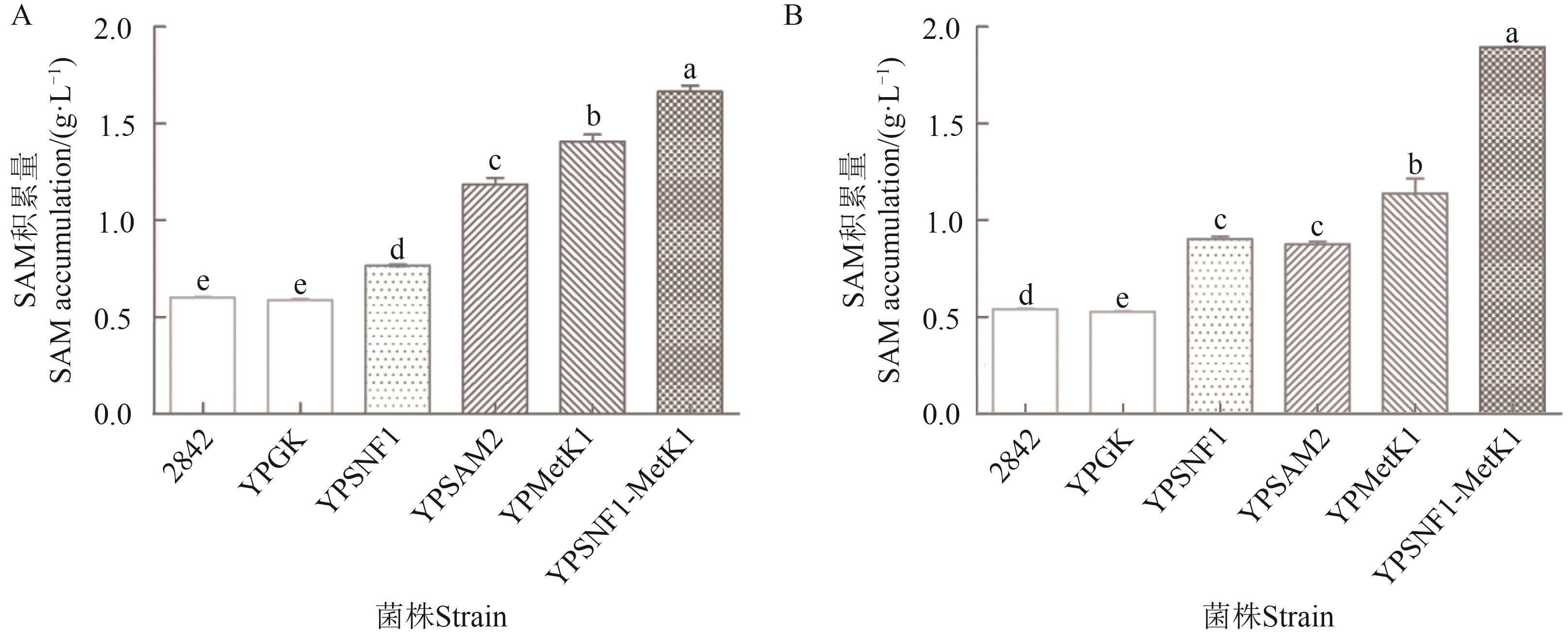
Fig. 2 SAM production of different strains in yeast cellA: 5% glucose; B: 10% glucose. Different lowercase letters indicate significant difference between different strains at P<0.05 level
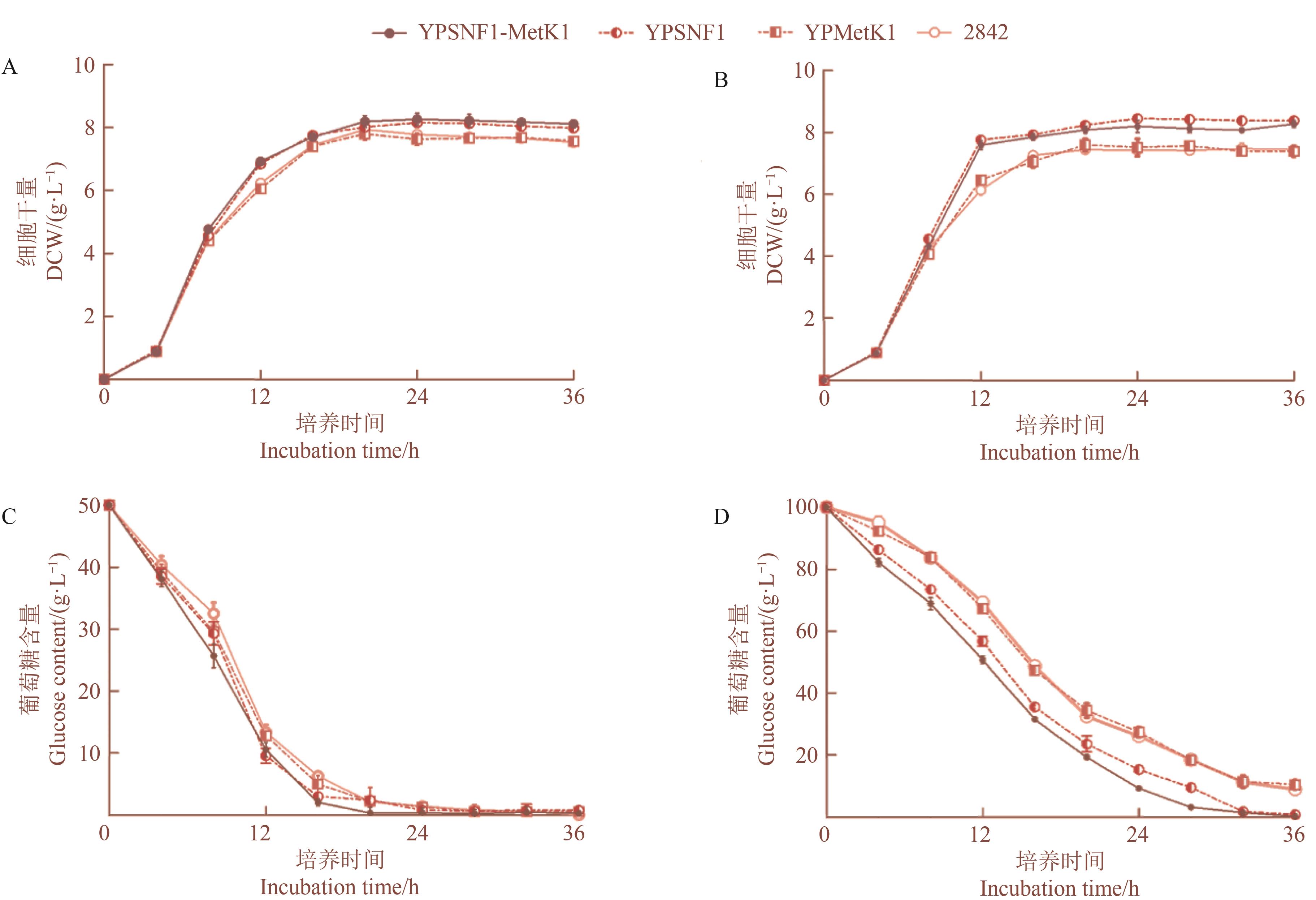
Fig. 3 Effects of SNF1 and MetK1 overexpression on growth and the glucose utilization of yeastA~B: Growth curves in 5% and 10% glucose, respectively; C~D: Glucose consumptions in 5% and 10% glucose, respectively
| 指标Index | 菌株Strain | |||
|---|---|---|---|---|
| 2842 | YPMetK1 | YPSNF1 | YPSNF1-MetK1 | |
SAM 含量 SAM content/(g·L-1) | 0.54±0.01 d | 0.90±0.13 c | 1.14±0.02 b | 1.90±0.01 a |
细胞干重 DCW/(g·L-1) | 7.45±0.12 b | 7.38±0.17 b | 8.38±0.08 a | 8.27±0.11 a |
SAM对细胞干重得率 Yield of SAM on DCW/(mg·g-1) | 72.48 d | 121.95 c | 136.04 b | 229.75 a |
SAM对葡萄糖得率 Yield of SAM on glucose/(mg·g-1) | 5.40 d | 9.00 c | 11.40 b | 19.00 a |
Table 2 Effects of overexpression of SNF1 and MetK1 on glucose utilization and SAM yeild
| 指标Index | 菌株Strain | |||
|---|---|---|---|---|
| 2842 | YPMetK1 | YPSNF1 | YPSNF1-MetK1 | |
SAM 含量 SAM content/(g·L-1) | 0.54±0.01 d | 0.90±0.13 c | 1.14±0.02 b | 1.90±0.01 a |
细胞干重 DCW/(g·L-1) | 7.45±0.12 b | 7.38±0.17 b | 8.38±0.08 a | 8.27±0.11 a |
SAM对细胞干重得率 Yield of SAM on DCW/(mg·g-1) | 72.48 d | 121.95 c | 136.04 b | 229.75 a |
SAM对葡萄糖得率 Yield of SAM on glucose/(mg·g-1) | 5.40 d | 9.00 c | 11.40 b | 19.00 a |
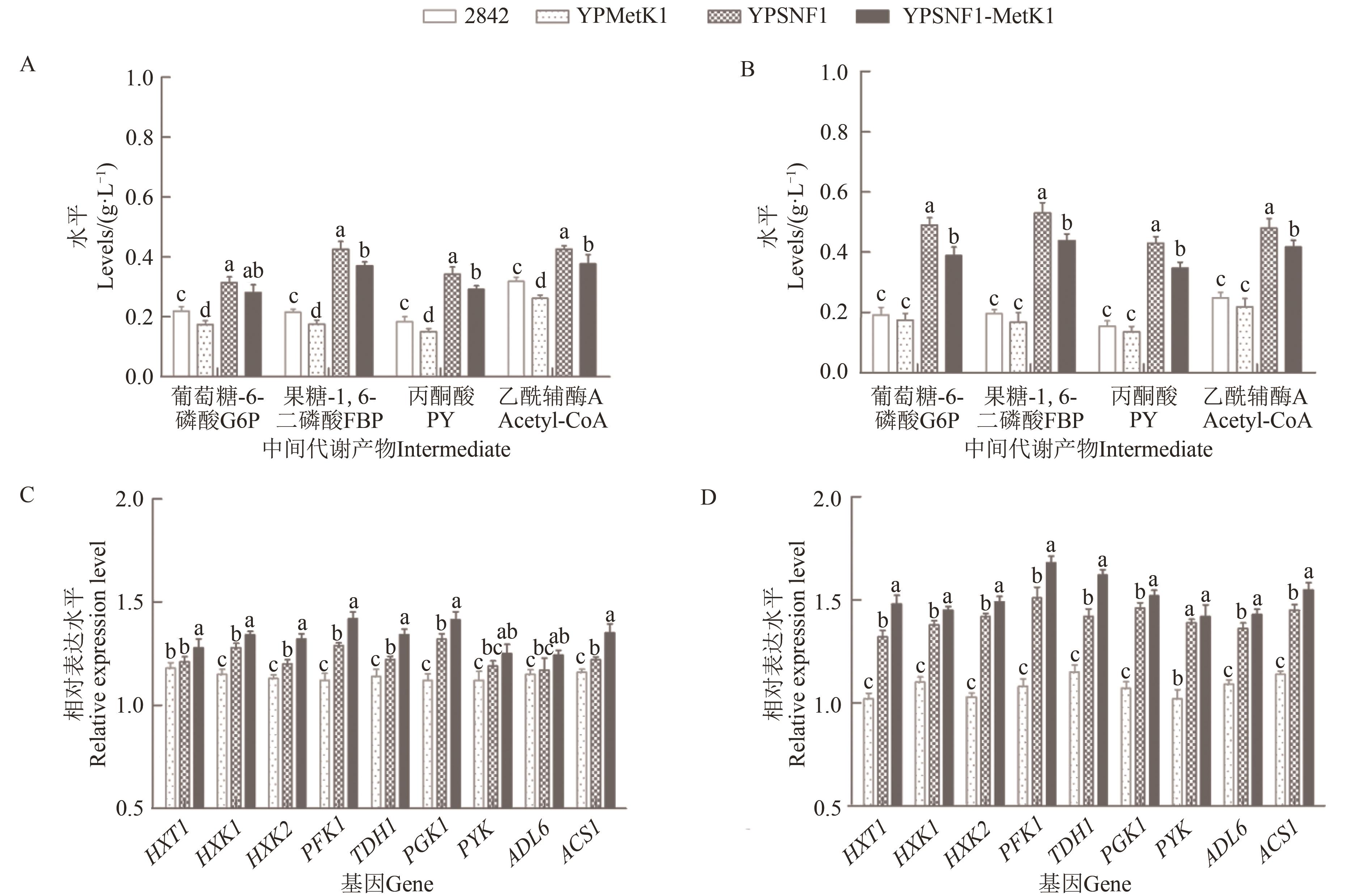
Fig. 4 Effects of SNF1 and MetK1 overexpression on glycolytic metabolism of yeastA~B: Levels of glycolytic intermediates in 5% and 10% glucose, respectively; C~D: Expression levels of genes related to glycolytic pathways in 5% and 10% glucose, respectively; different lowercase letters indicate significant differences between different strains at P<0.05 level

Fig. 5 Effects of SNF1 and MetK1 overexpression on precursor amino acid metabolism of yeastA~B: Precursor amino acids accumulations of strains in 5% and 10% glucose, respectively; C~D: Relative expression levels of genes related to amino acid metabolism of strains in 5% and 10% glucose, respectively; different lowercase letters indicate significant differences between different strains at P<0.05 level
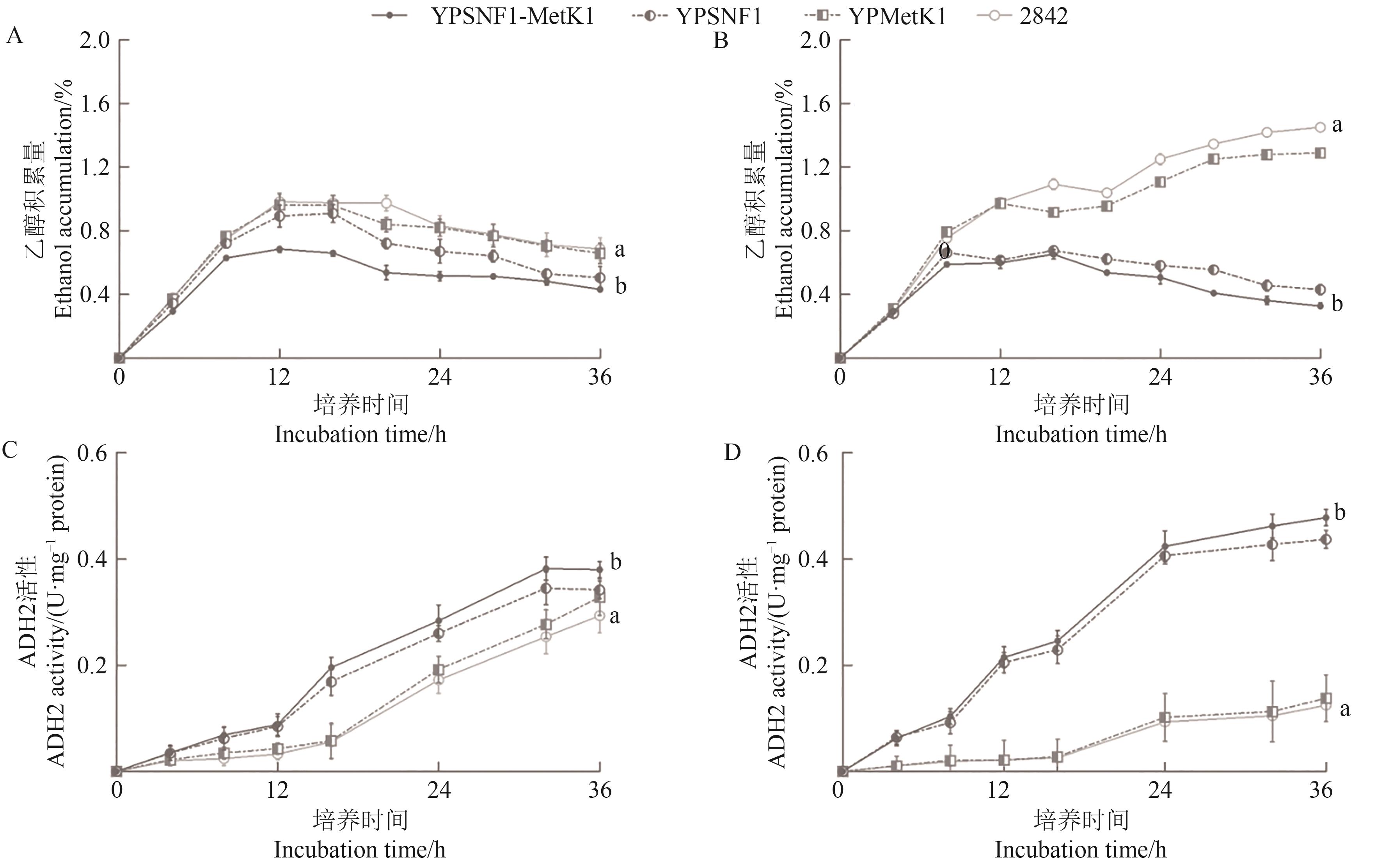
Fig. 6 Effects of SNF1 and MetK1 overexpression on ethanol metabolism of yeastA~B: Ethanol accumulation in 5% and 10% glucose, respectively; C~D: ADH2 activities in 5% and 10% glucose, respectively
| [1] | 陈海龙,蒋丽华,陈帅,等. Adk1过表达和柠檬酸钠补料促进酵母S-腺苷甲硫氨酸的合成[J].中国农业科技导报, 2020,22(10): 69-76. |
| CHEN H L, JIANG L H, CHEN S, et al.. Adk1 overexpression and sodium citrate feeding enhanced S-adenosylmethionine synthesis in yeast [J]. J. Agric. Sci. Technol., 2020, 22(10): 69-76. | |
| [2] | CEDERBAUM ARTHUR I. Hepatoprotective effects of S-adenosyl-L-methionine against alcohol-and cytochrome P450 2E1-induced liver injury [J]. World J. Gastroenterol., 2010, 16(11): 1366-1376. |
| [3] | CHEN H, WANG Z, CAI H, et al.. Progress in the microbial production of S-adenosyl-L-methionine [J/OL]. World J. Microbiol. Biotechnol., 2016, 32(9): 153 [2024-08-10]. . |
| [4] | LAN P, LI W, WEN T N, et al.. iTRAQ protein profile analysis of Arabidopsis roots reveals new aspects critical for iron homeostasis [J]. Plant Physiol., 2011, 155(2): 821-834. |
| [5] | MAYNE M B, COLEMAN J R, BLUMWALD E. Differential expression during drought conditioning of a root-specific S-adenosylmethionine synthetase from jack pine (Pinus banksiana Lamb.) seedlings [J]. Plant Cell Environ., 1996, 19(8): 958-966. |
| [6] | GONG B, WANG X, WEI M, et al.. Overexpression of S-adenosy-l-methionine synthetase 1 enhances tomato callus tolerance to alkali stress through polyamine and hydrogen peroxide cross-linked networks [J]. Plant Cell Tissue Organ Culture, 2016, 124(2): 377-391. |
| [7] | FUJIMOTO T, TOMITAKA Y, ABE H, et al.. Expression profile of jasmonic acid-induced genes and the induced resistance against the root-knot nematode (Meloidogyne incognita) in tomato plants (Solanum lycopersicum) after foliar treatment with methyl jasmonate [J]. J. Plant Physiol., 2011, 168(10): 1084-1097. |
| [8] | OWITI J, GROSSMANN J, GEHRIG P, et al.. iTRAQ-based analysis of changes in the cassava root proteome reveals pathways associated with post-harvest physiological deterioration [J]. Plant J., 2011, 67(1): 145-156. |
| [9] | 刘鑫, 李晓彤, 荆鑫, 等. S-腺苷甲硫氨酸对黄瓜断根扦插苗生长及生理代谢的影响[J].园艺学报, 2018, 45(8): 1513-1522. |
| LIU X, LI X T, JING X, et al.. Effect of S-adenosylmethionine on growth and physiological metabolism of cucumber cutting seedlings [J]. Acta Hortic. Sin., 2018, 45(8): 1513-1522. | |
| [10] | DAI Z, HUANG M, CHEN Y, et al.. Global rewiring of cellular metabolism renders Saccharomyces cerevisiae Crabtree negative [J/OL]. Nat. Commun., 2018, 9(1): 3059 [2024-08-10]. . |
| [11] | GAMBACORTA F V, DIETRICH J J, YAN Q, et al.. Rewiring yeast metabolism to synthesize products beyond ethanol [J]. Curr. Opin. Chem. Biol., 2020, 59: 182-192. |
| [12] | LIN J P, TIAN J, YOU J F, et al.. An effective strategy for the co-production of S-adenosyl-l-methionine and glutathione by fed-batch fermentation [J]. Biochem. Eng. J., 2004, 21(1): 19-25. |
| [13] | HEDBACKER K, CARLSON M. SNF1/AMPK pathways in yeast [J]. Front. Biosci., 2008, 13: 2408-2420. |
| [14] | COCCETTI P, NICASTRO R, TRIPODI F. Conventional and emerging roles of the energy sensor Snf1/AMPK in Saccharomyces cerevisiae [J]. Microb. Cell, 2018, 5(11): 482-494. |
| [15] | E-SCHOI, B-SPARK, LEE S-W, et al.. Increased production of S-adenosyl-L-methionine using recombinant Saccharomyces cerevisiae sake K6 [J]. Korean J. Chem. Eng., 2009, 26(1): 156-159. |
| [16] | HE J, DENG J, ZHENG Y, et al.. A synergistic effect on the production of S-adenosyl-L-methionine in Pichia pastoris by knocking in of S-adenosyl-L-methionine synthase and knocking out of cystathionine-beta synthase [J]. J. Biotechnol., 2006, 126(4): 519-527. |
| [17] | 周长林,杨明华,李登奎,等.一种高产腺苷蛋氨酸的菌种及其筛选方法: CN101481660B[P]. 2011-02-09. |
| [18] | CAO X T, YANG M H, DOU J, et al.. Strain improvement for enhanced production of S-adenosyl-L-methionine in Saccharomyces cerevisiae based on ethionine-resistance and SAM synthetase activity [J]. Ann. Microbiol., 2012, 62: 1395-1402. |
| [19] | CHEN H, YANG Y, WANG Z, et al.. Elevated intracellular acetyl-CoA availability by acs2 overexpression and mls1 deletion combined with metK1 introduction enhanced SAM accumulation in Saccharomyces cerevisiae [J]. Biochem. Eng. J., 2016, 107: 26-34. |
| [20] | ZHU A, ROMERO R, PETTY H R. An enzymatic colorimetric assay for glucose-6-phosphate [J]. Anal. Biochem., 2011, 419(2): 266-270. |
| [21] | DU Z J, WU W T. The repaid determination method of fructose-1,6-bisphosphate [J]. Chin. J. Biochem., 1993, 2: 59-62. |
| [22] | SAAVEDRA E, RAMOS-CASILLAS L E, MARÍN-HERNÁNDEZ A, et al.. Glycolysis in Ustilago maydis [J]. FEMS Yeast Res., 2008, 8(8): 1313-1323. |
| [23] | CHEN H, ZHU N, WANG Y, et al.. Increasing glycolysis by deletion of kcs1 and arg82 improved S-adenosyl-L-methionine production in Saccharomyces cerevisiae [J/OL]. AMB Express, 2021, 11(1): 20 [2024-08-10]. . |
| [24] | HAGISHITA T, YOSHIDA T, IZUMI Y, et al.. Efficient L-serine production from methanol and glycine by resting cells of Methylobacterium sp. strain MN43 [J]. Biosci. Biotechnol. Biochem., 1996, 60(10): 1604-1607. |
| [25] | ZHANG Y, XU H, LI S. A clean process for separating L-aspartic acid [J]. Chin. J. Bioprocess Eng., 2007, 5(4): 65-69. |
| [26] | KATRUSIAK A E, PATERSON P G, KAMENCIC H, et al.. Pre-column derivatization high-performance liquid chromatographic method for determination of cysteine, cysteinyl-glycine, homocysteine and glutathione in plasma and cell extracts [J]. J. Chromatogr. B Biomed. Sci. Appl., 2001, 758(2): 207-212. |
| [27] | 徐国强,陈修来,吴满珍.调控辅因子水平减少酿酒酵母积累副产物乙醇[J].食品与发酵工业, 2014, 40(10): 6-10. |
| XU G Q, CHEN X L, WU M Z. Reducing ethanol formation in Saccharomyces cerevisiae by regulating the level of cofactors [J]. Food Ferment. Ind., 2014, 40(10): 6-10. | |
| [28] | JOHANSSON M, SJÖSTRÖM J E. Enhanced production of glycerol in an alcohol dehydrogenase (ADH I) deficient mutant of Saccharomyces cerevisiae [J]. Biotechnol. Lett., 1984, 6(1): 49-54. |
| [29] | MAURICIO J C, MORENO J J, ORTEGA J M. In vitro specific activities of alcohol and aldehyde dehydrogenases from two flor yeasts during controlled wine aging [J]. J. Agric. Food Chem., 1997, 45(5): 1967-1971. |
| [30] | MENG L, LIU H L, LIN X, et al.. Enhanced multi-stress tolerance and glucose utilization of Saccharomyces cerevisiae by overexpression of the SNF1 gene and varied beta isoform of Snf1 dominates in stresses [J/OL]. Microb. Cell Fact., 2020, 19(1): 134 [2024-08-10]. . |
| [31] | YUAN J S, REED A, CHEN F, et al.. Statistical analysis of real-time PCR data [J/OL]. BMC Bioinformatics, 2006, 7: 85 [2024-08-10]. . |
| [32] | LI Y, ZHANG Y, YE D, et al.. Impact of serine and serine synthesis genes on H2S release in Saccharomyces cerevisiae during wine fermentation [J/OL]. Food Microbiol., 2022, 103: 103961 [2024-08-10]. . |
| [33] | OUD B, C-LFLORES, GANCEDO C, et al.. An internal deletion in MTH1 enables growth on glucose of pyruvate-decarboxylase negative, non-fermentative Saccharomyces cerevisiae [J/OL]. Microb. Cell Fact., 2012, 11: 131 [2024-08-10]. . |
| [34] | IDA Y, FURUSAWA C, HIRASAWA T, et al.. Stable disruption of ethanol production by deletion of the genes encoding alcohol dehydrogenase isozymes in Saccharomyces cerevisiae [J]. J. Biosci. Bioeng., 2012, 113(2): 192-195. |
| [35] | VAN MARIS A J A, GEERTMAN J A, VERMEULEN A, et al.. Directed evolution of pyruvate decarboxylase-negative Saccharomyces cerevisiae, yielding a C2-independent, glucose-tolerant, and pyruvate-hyperproducing yeast [J]. Appl. Environ. Microbiol., 2004, 70(1): 159-166. |
| [36] | 白逢彦.走出中国:酿酒酵母的起源、驯养与演化[J].微生物学报, 2023, 63(5): 1748-1770. |
| BAI F Y. Out of China: origin, domestication and evolution of Saccharomyces cerevisiae [J]. Acta Microbiol. Sin., 2023, 63(5): 1748-1770. | |
| [37] | HONG S P, LEIPER F C, WOODS A, et al.. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases [J]. Proc. Natl. Acad. Sci. USA, 2003, 100(15): 8839-8843. |
| [38] | LIN X, ZHANG C Y, BAI X W, et al.. Effects of GLC7 and REG1 deletion on maltose metabolism and leavening ability of baker’s yeast in lean dough [J]. J. Biotechnol., 2015, 209: 1-6. |
| [39] | SCHÜLLER H J. Transcriptional control of nonfermentative metabolism in the yeast Saccharomyces cerevisiae [J]. Curr. Genet., 2003, 43(3): 139-160. |
| [40] | VORONKOVA V, KACHEROVSKY N, TACHIBANA C, et al.. Snf1-dependent and Snf1-independent pathways of constitutive ADH2 expression in Saccharomyces cerevisiae [J]. Genetics, 2006, 172(4): 2123-2138. |
| [1] | Yijun WANG, Huoqing HUANG, Xiaoyun SU. Construction of Bifunctional Saccharomyces cerevisiae with Ability to Detoxify Mycotoxins [J]. Journal of Agricultural Science and Technology, 2024, 26(2): 226-233. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号